S-layer proteins as basic building blocks in a biomolecular construction kit
by
Dietmar Pum*, Angela Neubauer, Erika Gyoervary, Margit Sára, Uwe B. Sleytr
Center for Ultrastructure Research and Ludwig Boltzmann Institute for Molecular Nanotechnology
Universität für Bodenkultur Wien, Vienna, Austria
*[email protected]
This is a draft paper for the
Seventh
Foresight Conference on Molecular Nanotechnology.
The final version has been submitted
for publication in the special Conference issue of Nanotechnology.
Abstract
Crystalline bacterial cell surface layer (S-layer) proteins have been optimized during billions of years of biological evolution as constituent elements of one of the simplest self-assembly systems. Isolated S-layer proteins have the intrinsic property to recrystallize into two-dimensional arrays at a broad spectrum of surfaces (e.g. silicon) and interfaces (e.g. planar lipid films). The well defined arrangement of functional groups on S-layer lattices allows the binding of molecules and particles in defined regular arrays. S-layers recrystallized on solid supports can be patterned in the submicrometer range using standard optical lithography. S-layers also represent templates for the formation of inorganic nanocrystal superlattices (e.g. CdS, Au, Ni, Pt, or Pd) as required for molecular electronics and non-linear optics.
1. Introduction
The current challenge in the trend of exponential miniaturization, known as Moores' law, is the search for a viable successor for existing microlithographic procedures. Although the industry is still investigating new techniques essentially based on (deep) ultraviolet , x-ray proximity or ion-beam projection lithography, it has become yet clear that the development of nano-scale systems will require entirely new materials, processes, and device architectures [TenWolde 1998]. In particular, the ultimately high requirements in positional control at the nanometer scale, and the synthesis and assembly of molecular functional units can only be met when novel concepts and techniques originating from biotechnology, organic chemistry, scanning probe microscopy and solid-state physics are used. These concepts are essentially based on bottom-up approaches where self-assembly and molecular recognition play a key role. Self-assembly is a basic building principle in nature for generating large arrays of biomolecules with well-defined geometrical and physicochemical surface properties. In the development of functional "nanoscale" devices, the application of self-assembly for achieving perfect positional control at the molecular level appears feasible, both theoretically and experimentally, and offers striking advantages in manufacturing processes.
Crystalline bacterial cell surface layer (S-layer) proteins have been optimized during billions of years of biological evolution as building blocks of one of the simplest self-assembly systems. [Pum and Sleytr 1999, Sleytr et al. 1999, 2000]. This is due to the fact that S-layer proteins have the intrinsic property to reassemble into two-dimensional arrays on surfaces of a broad spectrum of materials (e.g. silicon wafers, metals, polymers) and interfaces (e.g. planar lipid films or liposomes), the arrangement of functional domains on each S-layer unit cell is repeated with the periodicity of the S-layer lattice at a distance of approximately 10 nm, enabling the formation of regular arrays of bound molecules and particles.
2. Characteristic properties of S-layer proteins
S-layer proteins form the outermost cell envelope component of a broad spectrum of bacteria and archaea (Fig.1) [for compilation see Sleytr et al. 1996]. S-layers are composed of a single protein or glycoprotein species (Mw 40-200 kDa) and exhibit either oblique (p1, p2), square (p4) or hexagonal (p3, p6) lattice symmetry with unit cell dimensions in the range of 3 to 30 nm. S-layers are generally 5 to 10 nm thick and show pores of identical size (diameter, 2 - 8 nm) and morphology [Sleytr et al. 1996, 1999].
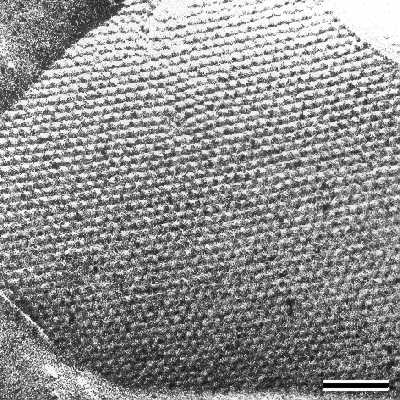 |
| Fig.1 Transmission electron micrograph of a freeze-etched, metal shadowed preparation of a bacterial cell with an S-layer with hexagonal lattice symmetry. Bar, 100nm |
S-layer proteins show a remarkably difference in surface corrugation and chemistry between inner and outer face. The topography of the inner face (with respect to the bacterial cell) is more corrugated than to outer one. This has been demonstrated by comparative electron microscopical studies of freeze-etched and metal shadowed bacterial cells, of freeze-dried metal shadowed S-layer fragments, and by digital processing of electron micrographs from tilt series recorded in a transmission electron microscope [Baumeister and Engelhardt 1987, Hovmöller et al. 1988, Beveridge 1994] and scanning force microscopy of recrystallized S-protein monolayers [Pum and Sleytr 1995a, Müller et al. 1996, 1997, 1998, Engel 1997]. Labeling experiments with electron microscopical marker molecules, such as positively charged polycationic ferritin (PCF; diameter approximately 12 nm) and chemical modification of the surface properties showed for S-layers of most Bacillaceae a net negatively charged inner and a charge neutral outer face [Sara and Sleytr 1989]. The net negative surface charge on the inner face is caused by an excess of carboxyl groups while on the outer face carboxyl groups and amino groups are found in equimolar amounts.
In most S-layer lattices the constituent subunits interact with each other and with the supporting cell envelope layer (e.g. plasma membrane, outer membrane or peptidoglycan) by a combination of non-covalent forces including hydrogen or ionic bonds, and hydrophobic or electrostatic interactions [Sleytr and Messner 1983]. Since S-layer proteins are composed of a high proportion of non-polar amino acids hydrophobic interactions are expected to play a particularly important role in the assembly process [Sleytr and Messner 1989, Sleytr et al. 2000]. Further, studies on the distribution of charged groups on S-layer lattices of Bacillaceae have shown that free amino and carboxyl groups of adjacent protomers are arranged in close proximity and thus contribute to the stability of the array by electrostatic interactions [Sleytr and Messner 1989, Sara and Egelseer 1996]. In particular, isolation and disintegration experiments with S-layer lattices clearly demonstrated that the intermolecular forces between the subunits are stronger than those binding the crystalline array to the supporting envelope layer [Sleytr 1975]. Studies on the strucure-function relationship of different S-layers from Bacillaceae revealed the existence of specific (lectine type) binding domains on the N-terminal part of the S-layer proteins for secondary cell wall polymers covalently linked to the peptidoglycan matrix of the cell wall [Ilk et al. 1999]. This properties are seen as a basic requirement for the dynamic recrystallisation and reorganisation process of the S-layer lattice on the bacterial cell surface in the course of cell growth and cell division. One of the most fascinating properties of S-layer proteins is their capability to reassemble in suspension [Sleytr and Messner 1989], at the liquid-air interface [Pum et al. 1993], at solid surfaces [Pum et al. 1995a], at floating lipid monolayers [Pum et al. 1993, Pum and Sleytr 1994] and on liposomes [Küpcü et al. 1995] (Fig.2). The formation of monolayers at surfaces and interfaces is a key feature of S-layers in a broad spectrum of nanobiotechnological applications [for review see Pum and Sleytr 1999, Sleytr et al. 2000].
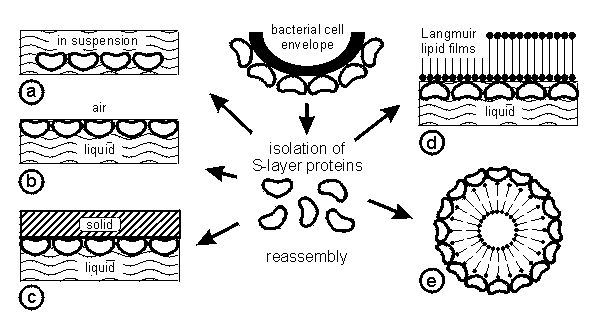 |
| Fig.2 Schematic drawing of the isolation of S-layer proteins from bacterial cells and their reassembly into crystalline arrays in suspension (a), at solid supports (b), at the air-water interface (c), on lipid films (d) and on liposomes (e). The orientation of the recrystallized lattice is determined by the physicochemical properties of the surfaces. |
3. Reassembly in suspension
S-layer proteins have shown the intrinsic tendency to reassemble into two-dimensional arrays after removal of the disrupting agent used in the dissolution procedure [Sleytr and Messner 1989, Sleytr et al. 2000]. In general, a complete disintegration of S-layer lattices on bacterial cells can be achieved using high concentrations of chaotropic agents (e.g. guanidine hydrochloride, urea), by lowering or raising the pH value, or by applying metal-chelating agents (e.g. EDTA, EGTA) or cation substitution. After removal of the disrupting agent, the S-layer proteins reassemble into flat sheets, open ended cylinders or closed vesicles (Fig.3).
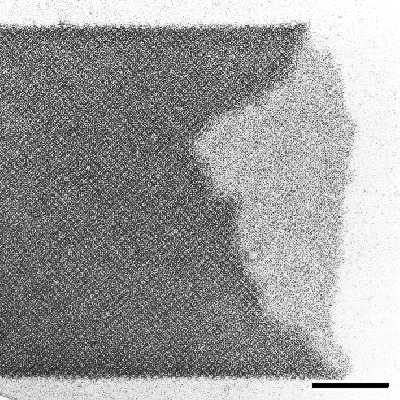 |
| Fig.3 Transmission electron micrograph of negatively stained preparation of a cylindrical S-layer self-assembly product. Bar, 200nm |
Structural form and size of the self-assembly products depend on the particular S-layer protein species used and also on several environmental parameters such as temperature, pH value, ion composition and/or ionic strength. Studies on self-assembly processes have shown that the initial phase is determined by a rapid nucleation of the subunits into oligomeric precursors consisting of several unit cells. Subsequently, these aggregates reassemble in a much slower process into larger crystalline arrays [Jaenicke et al. 1985]. The self-assembly properties are determined by the amino acid sequence of the polypeptide chains and consequently the tertiary structure of the S-layer protein species [Sleytr 1975]. It must also be noted here that often double layers are formed in the course of the self-assembly process. In this particular case the two constituent monolayers are facing each other either with their inner or their outer side. Double layers are often only stabilized by divalent cations interacting with polar groups [Messner et al. 1986a].
4. Recrystallization at interfaces (liquid-air, liquid-solid) and on lipid films
Reassembly at the air-water interface, at solid supports and on lipid films has proven to be a very powerful way to generate extended closed S-layer protein monolayers. As determined by electron and scanning force microscopical studies, the reassembly starts at several distant nucleation points at the surface and proceeds in-plane until neighbouring crystalline areas meet [Pum et al. 1993, Pum and Sleytr 1995a]. In this way, a closed mosaic of randomly aligned monocrystalline S-layer domains is formed. Size and shape of the individual domains depend on the particular S-layer proteins used and on the environmental conditions of the so called "crystallization solution" which comprises the buffer and the appropriate amount of solubilized S-layer protein.
4.1 Liquid-air interface
The importance of ionic compositon and strength in the crystallization solution for the formation of extended crystalline arrays at the liquid-air interface was demonstrated for the reassembly of the S-layer protein of Bacillus sphaericus CCM2177 [Pum and Sleytr 1995b]. Depending on the calcium concentration in the crystallisation solution a broad spectrum of morphologies ranging from tenuous, fractal-like to large monocrystalline domains was obtained [Pum and Sleytr 1994, 1995b]. The optimum ion concentration for the crystallisation solution, determined for obtaining coherent lattices, was 10 mM CaCl2 . Since equivalent results were obtained with other bivalent cations such as Ba2+ or Mg2+ it was concluded that salt-bridges are required for the intermolecular binding of the S-layer subunits. On the other hand monovalent ions are not required for the S-layer assembly process. Under optimum conditions large-scale domains of crystalline arrays could be observed at the water-air interface already within a few hours after beginning of the recrystallization process. Due to the asymmetry in the surface properties of the S-layer lattices all crystalline domains had the same orientation with respect to the interface [Pum et al. 1993, Pum and Sleytr 1994].
4.2 Liquid-solid interface
The intrinsic property of S-layer proteins to form extended crystalline arrays on solid supports is one of the most important features for functionalizing surfaces. The recrystallisation of S-layer proteins on solid substrates (see Table 1) has been studied by atomic force (AFM) and transmission electron microscopy (TEM).
Silicon wafers:
( Orientation <100> and <111> ) |
- with native oxide layer
- silanized with
- Octadecyltrichlorosilane (OTS)
- 3-(trimethoxysilyl)propyl-methacrylate (TPS)
- Decyldimethylsilane (DMS)
- Hexamethyldisilane (HMDS)
- Dimethyloctadecylchlorosilane
- 2-Aminopropyltrimethoxysilane
- 3-Mercaptopropyltriethoxysilane
- coated with photoresist (Type AZ 1350, Hoechst AG, Germany)
- coated with a carbon film
|
Silicon nitride, gallium arsenide wafers
Noble metals (e. g. gold, platinum or titan)
Glass, Cellulose, Polymers, Graphite, Highly oriented pyrolytic graphite (HOPG), Mica |
| Table 1: Substrates used in the formation of S-layer protein monolayers |
These experiments demonstrated that the formation of coherent crystalline arrays strongly depend on the specific S-layer protein used, on the conditions of the crystallization solution and on the surface properties of the substrate. For example, the size of individual monocrystalline domains obtained from S-layer proteins from B. coagulans E38-66 may be as large as 10-20 µm in diameter while S-layer protein from B. sphaericus CCM2177 forms much smaller crystalline domains (Fig.4).
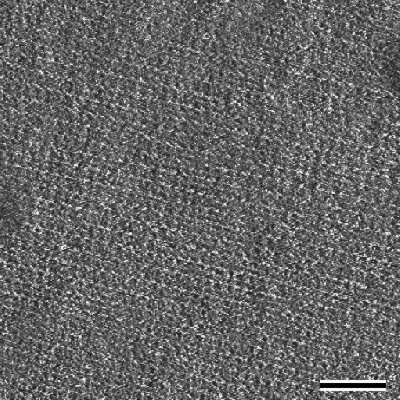 |
| Fig.4 Scanning force microscopical image of a S-layer lattice with oblique lattice symmetry recrystallized on the surface of a silicon wafer. This image was obtained in contact mode under water. Bar, 50nm |
Concerning the surface properties of the substrate, hydrophobic surface characteristics are most often required for crystal growth although exceptions to this rule could be observed. For example, S-layer protein monolayers of B. stearothermophilus NRS 2004/3a [Pum and Sleytr 1995a] and B. stearothermophilus PV72/p2 (unpublished results) were only formed on substrates with hydrophobic surfaces (e.g. silicon wafers with a native oxide layer or coated with polymeric resists) [Pum and Sleytr 1995a; Sleytr et al. 1997]. In these situations the S-layer protein monolayer is oriented with its outer more hydrophobic face against the hydrophobic support. In contrast, large monocrystalline domains of B. sphaericus CCM2177 were also formed on hydrophilic surfaces (unpublished data). These observations could be confirmed with recrystallization experiments with S-layer protein from B. coagulans E38-66 which reassembled with its outer more hydrophobic face on poly-L-lysine (PLL) coated grids [Pum et al. 1989].
AFM measurements with chemically-functionalized probes indicated repulsive surface forces between S-layer protein monolayers and both hydrophilic and hydrophobic probes (paper in preparation). These repulsive forces were interpreted to be steric forces arising from the presence of solvated macromolecular chains protruding from the S-layer surface. Possible macromolecules in the S-layers are secondary cell wall polymers (SCWP) involved in the specific binding of S-layer proteins to the supporting peptidoglycan containing layer and remaining attached to the solubilized S-layer protein [Ries et al. 1997, Ilk et al. 1999]. Preliminary studies have also shown that SCWPs can be used to facilitate or support the recrystallisation of S-layer proteins which otherwise would not form monocrystalline layers on solid substrates by themselves (paper in preparation).
4.3 S-layers as stabilizing structures for solid-supported lipid membranes
Currently the development of solid supported functionalized lipid membranes attracts considerable attention in the development of a new generation of highly specific and sensitive biosensors. In particular, the increase of the long-term robustness is of major interest. Thus, it was recently proposed to place soft polymer cushions between substrate and functionlized lipid film in order to maintain the thermodynamic and structural properties of the lipid membrane [Sackman 1996]. Further, this polymeric layer should also act as functional ionic reservoir space, and provide an environment for the incorporation of proteins under non-denaturing conditions in a well-defined orientation. As an alternative to this approach, lipid-bilayer and tetraetherlipid-monolayer films on solid supports can also be stabilized by S-layers. As previously demonstrated, in comparison with unsupported mono- or bimolecular lipid layers, lipid films associated with S-layers are much more stable structures [Pum and Sleytr 1994, Schuster et al. 1998a,b, Wetzer et al. 1997]. The terminology 'semifluid membranes' has been used to describe S-layer-supported membranes since the interaction of the lipid head groups with the repetitive domains of the associated S-layer lattice significantly modulates the characteristics of the lipid film (particularly its fluidity and local order on the nanometer scale). Fluorescence recovery after photobleaching (FRAP) measurements demonstrated that the mobility of lipids in S-layer-supported bilayers was higher than in other model systems, such as hybrid bilayers or dextran-supported bilayers [Györvary et al. 1999]. Further, it has also been shown that in comparison to plain lipid membranes S-layer supported lipid membranes had a reduced tendency to rupture, especially in the presence of ionophores [Schuster et al. 1998a] or pore forming proteins [Schuster et al. 1998b]. Based on these experiments, studies are in progress to exploit the functionality of transmembrane proteins in S-layer stabilized solid-supported lipid membranes.
4.4 Lipid films
Electron microscopical [Pum et al. 1993] and scanning force microscopical [Wetzer et al. 1997] studies have shown that S-layer protein subunits reassemble into closed, polycrystalline monolayers on a great variety of phospholipid films [Wetzer et al. 1998].
In order to obtain more detailed information on the influence of the phase state of the lipid monolayer on the recrystallization process different surface analytical techniques including dual label fluorescence microscopy [Diederich et al. 1996], FTIR spectroscopy, X-ray reflectometry, grazing incidence X-ray diffraction (GIXD), X-ray and neutron scattering [Weygand 1999a, b] were used. It was shown that the S-layer proteins initially adsorb (without interpenetrating the monolayer) more effectively under the disordered, liquid expanded phase than under the ordered, liquid condensed phase. The adsorption was dominated by hydrophobic and van der Waals interactions. Further, the two-dimensional protein crystals grew preferentially under the ordered lipid domains followed by an overcoating of the disordered lipid areas after prolonged periods of time [Diederich et al. 1996]. FTIR studies have shown that the S-layer protein when bound to the lipid headgroups affected the segmental order of the hydrophobic lipid chains. Steric forces are believed to be responsible for the different recrystallization behaviour in respect to the phase state of the lipid monolayer.
Beside, the phase state of the lipid film, the nature of the lipid headgroup also affects the formation of the S-layer lattice at the lipid film. From an extensive recrystallization study with the S-layer protein from B. coagulans E38-66 at diverse lipid monolayers [Wetzer et al. 1998] it was concluded that S-layers are attached to lipid monolayers with their outer face as the buffer pH approaches the pI of the protein (an exception: no recrystallization under acidic lipid films on pure water at pH=4). Conversely, the S-layer was oriented with its inner face towards the lipid monolayer when the pH value of the buffer was in the range of 5.7-9 ( with or without Ca2+- ions). An exception to this rule was observed with one cationic lipid film at pH=5.7 where the S-layer lattice was associated with its outer face towards the lipid layer [Wetzer et al. 1998]. Based on these results it was concluded that the recrystallization process and the structure of the lipid/S-layer composite film depends on the nature of the lipid head group, the phase state of the lipid film, the ionic content and pH value of the subphase.
5. Lithographic patterning of S-layers by deep-UV (DUV) irradiation
Patterning of S-layers recrystallized on solid substrates is an essential requirement for many applications in nanotechnology and supramolecular engineering. In previous studies it was demonstrated that S-layers can be patterned using deep ultraviolet (DUV) radiation [Pum et al. 1996, 1997]. S-layers on silicon wafers are brought into direct contact with a photomask (synthetic quartz glass with a patterned 100nm thick chromium coating) and exposed to DUV irradiation (ArF; 193 nm, dose ca. 100 mJ cm-2 per pulse; pulse duration ca. 8 nsec) (Fig.5).
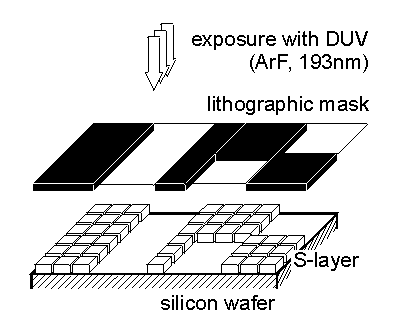 |
| Fig.5 Schematic drawing of the patterning of an S-layer on a silicon wafer by deep ultraviolet radiation (DUV) using an ArF excimer laser. |
AFM investigations of DUV patterned S-layers revealed that the S-layer was completely removed from the silicon substrate in the exposed areas at a dosage of 200 mJ cm-2. The pattern on the photomask which consisted of lines and squares (feature sizes ranging from 200 nm to 1000 nm) with different line-and-space ratios was transferred into the S-layer (Fig.6).
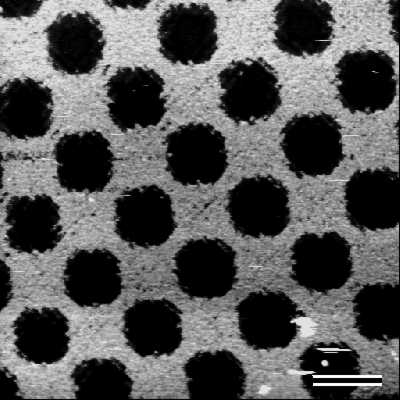 |
| Fig.6 Scanning force microscopical image of a patterned S-layer on a silicon wafer. Bar, 1000nm |
Patterning experiments were also carried out using KrF laser radiation (wavelength 248 nm); emitted after excitation of krypton-fluoride gas in an excimer laser (dose ca. 350 mJ cm-2 per pulse; pulse duration ca. 12 nsec). The thickness of the S-layer in the exposed areas was reduced significantly compared to unexposed regions but it was clear from AFM height measurements that the protein was not removed completely, even after increasing the dosage up to 10 laser pulses of approximately 350 mJ cm-2 each. It was concluded from this observation that the S-layer protein absorbed far less at the KrF wavelength compared to the ArF line and therefore the energy of the photons at the wavelenght of 248 nm was not sufficient to crack intramolecular bonds in the S-layer protein. Consequently, S-layers were thermally modified (carbonized) but not ablated by KrF irradiation [Pum et al. 1997]. Preliminary results from immobilization experiments on DUV-patterned S-layers indicated that in the unexposed regions the S-layers maintained their functional integrity. This has been shown either by binding fluorescent markers onto patterned S-layers or by reinforcing the protein layer with 2,2,4,4,6,6 Hexamethylcyclotrisilazan using a silylation procedure [Shaw et al. 1989] and subsequent reactive ion etching (unpublished results). S-layers have also been suggested as natural resists with nanometer resolution for "at-the-surface-imaging" in micro-and nanoelectronical applications. Since S-layers are only 4-10 nm thick and consequently much thinner than conventional resists, considerable improvement in edge resolution of submicron structures can be expected.
6. Binding of functional molecules
Controlled immobilization of functional molecules on surfaces is an essential requirement in most areas of supramolecular engineering. However, the assembly of molecular or atomic devices generally depends on a geometrically and physicochemically precisely defined, regular immobilization matrix which in its basic structural scale corresponds to the size of the respective molecules or atoms [Avouris 1995]. Eigler and coworkers were the first to demonstrate this at the atomic level by placing atoms on the atomic lattice of solid metal surfaces [Eigler and Schweitzer 1990]. Accordingly, protein lattices such as S-layers with unit cell dimensions in the nanometer range are perfectly suited for the manipulation and controlled immobilization of biologically active (macro)molecules. This is because the well defined position, orientation, and, in particular, high density of functional groups (about 1.6 x 106 carboxyl groups per µm2 ) allows that molecules can be immobilized as dense monolayers resembling the pattern of the underlying lattice. The immobilization may be either performed by covalent or non-covalent bonds.
In the last years, S-layer research had a strong focus on the development of biosensors [Neubauer et al. 1994, 1996, Taga et al. 1994] and dipstick style immunoassays [Breitwieser et al. 1996, 1998]. In the course of these studies, a whole spectrum of functional macromolecules including enzymes, antibodies, antigens and ligands has already been covalently immobilized to different S-layer matrices (S-layer carrying cell wall fragments, S-layer ultrafiltration membranes or recrystallized S-layer protein monolayers) [Sára and Sleytr 1989, Weiner et al. 1994, Küpcü et al. 1996]. Usually, the S-layer lattice was crosslinked with glutardialdehyde before the free carboxyl groups were activated with 1-ethyl-3,3'(dimethylamino)propylcarbodiimide (EDC). The activated carboxyl groups could then react with free amino groups of foreign macromolecules leading to stable peptide bonds beween the S-layer matrix and the immobilized protein molecules [Sára and Sleytr 1989].
Specific binding of molecules on S-layer lattices may also be induced by different non-covalent forces. The pattern of binding frequently reflect the lattice type, the size of the morphological units and the distribution of physicochemical properties on the array [Messner et al. 1986b, Sára et al. 1996]. For example, positively charged polycationic ferritin (PCF) was immobilized to the S-layer lattice of B. coagulans E38-66 in a way that the PCF molecules reflected the distribution of net negatively charged sites on the S-layer lattice (Fig. 7).
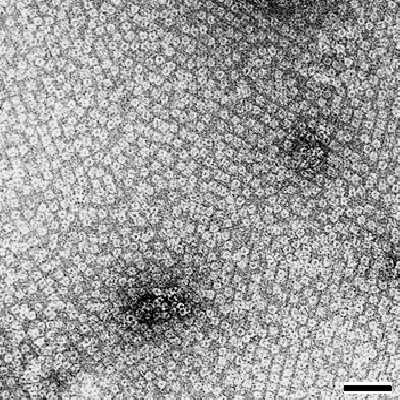 |
| Fig.7 Transmission electron micrograph of polycationic ferritin molecules regularly bound to the crystalline domains of an S-layer with oblique lattice symmetry. The grain boundaries between the individual patches are seen as line imperfections. Bar, 50nm |
7. Formation of inorganic nanocrystal superlattices on S-layers
Inorganic nanoparticles are a new generation of materials with novel chemical and physical properties usually not available in bulk materials [Alivisatos 1996]. Presently liquid phase synthesis by selective precipitation or Langmuir-Blodgett techniques are most often used in the generation of nanoparticles. As an alternative to these methods S-layers can be used as periodic nanometric templates in the nucleation of inorganic nanoparticles into ordered arrays. Recently, it was demonstrated that S-layer proteins recrystallized on solid supports or S-layer self-assembly products which were deposited on such supports may be used to induce the formation of CdS particles [Shenton et al. 1997] or gold nanoparticles [Dieluweit et al. 1998]. Inorganic superlattices of CdS with either oblique or square lattice symmetries were fabricated by exposing self-assembled S-layer lattices to Cd- ion solutions followed by slow reaction with hydrogen sulfide. Precipitation of the inorganic phase was confined to the pores of the S-layers with the result that CdS superlattices with prescribed symmetries were prepared. In a similar procedure a square superlattice of uniform 4 to 5 nm sized gold particles with 12.8 nm repeat distance was fabricated by exposing a square S-layer lattice with preinduced thiol groups to a tetrachloroauric (III) acid solution [Dieluweit et al. 1998]. Transmission electron microscopical studies showed that the gold nanoparticles were formed in the pore region during electron irradiation of an initially grainy gold coating covering the whole S-layer lattice. The shape of the gold particles resembled the morphology of the pore region of the square S-layer lattice. By electron diffraction and energy dispersive X-ray analysis the crystallites were identified as gold (Au(0)). Electron diffraction patterns revealed that the gold nanoparticles were crystalline but in the long range order not crystallographically aligned. These experiments were repeated with a broad range of different metal salts such as PdCl2, NiSO4, KPtCl6, Pb(NO3)2, and K3Fe(CN)6 (Fig.8). These experiments have clearly demonstrated that nanocrystal superlattices can be induced by S-layers as templates with a broad range of particle sizes ( 5 to 15 nm in diameter), interparticle spacings (up to 30 nm) and lattice symmetries (oblique, square or hexagonal).
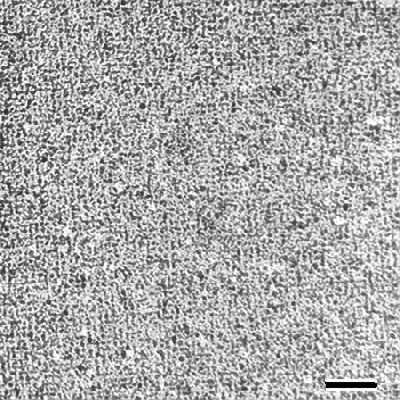 |
| Fig.8 Transmission electron micrograph of palladium (Pd) nanoparticles precipitated on a S-layer with square lattice symmetry. Bar, 20nm |
8. Conclusions
The use of S-layer proteins as constituent elements for fabricating supramolecular structures is new in the field of molecular nanotechnology. It is a simple and at the same time fascinating construction principle where the properties of a single protein (subunit) define the functional characteristics of a whole two-dimensional array. Detailed experiments with biologically functional molecules, such as antibodies, enzymes, and ligands have demonstrated the high binding capacity of S-layers. Spatial control in the submicrometer range can be obtained by the application of deep ultraviolet. Due to the crystalline character of S-layers a well defined mean spacing of deposited metal particles can be achieved which is necessary for a broad range of electronic or optical applications. S-layers can also be used as an alternative to soft polymer cushions, to stabilize and support functional lipid membranes. This supramolecular design was optimized by archaea which dwell under the most extreme environmental conditions. Finally, it is expected that, in the near future, modifications of S-layer proteins by recombinant DNA technologies will significantly influence the development of applied S-layer research
So far, basic and applied S-layer research has demonstrated that nature can be considered as the ultimate example of how to construct nanometer size, molecular self assembly systems. The remarkable intrinsic features of S-layer proteins and the possibility for combining S-layer lattices with other functional molecules in a spatial predictable way make them to unique elements in molecular nanotechnology and biomimetics.
Acknowledgements
Part of this work was supported by the Austrian Science Foundation (FWF) project S72 and the Austrian Federal Ministry of Science and Transport.
References
- Alivisatos A P 1996 Science 271 933
- Avouris P 1995 Account. Chem. Res. 28 95-102
- Baumeister W, Engelhardt H 1987 Three-dimensional structure of bacterial surface layers (Electron microscopy of proteins) ed J R Harris, R W Horne (Vol 6 London: Academic Press) p 109
- Beveridge T J 1994 Curr. Opin Struct Biol 4 204-12
- Breitwieser A, Küpcü S, Howorka S, Weigert S, Langer C, Hoffmann-Sommergruber K, Scheiner O, Sleytr U B, Sára M 1996 BioTechniques 21 918-25
- Breitwieser A, Mader C, Schocher I, Hoffmann-Sommergruber K, Aberer W, Scheiner O, Sleytr U B, Sára M 1998 Allergy 53 786-93
- Diederich A, Sponer C, Pum D, Sleytr U B, Lösche M 1996 Colloids Surf. B: Biointerf. 6 335-46
- Dieluweit S, Pum D, Sleytr U B 1998 Supramolec Sci 5 15-19
- Engel A, Schoenenberger C A, Müller D J 1997 Curr Opin Struct Biol 7 279-84
- Eigler D M, Schweizer E K 1990 Nature 344 524-26
- Györvary E, Wetzer B, Sleytr U B, Sinner A, Offenhäusser A, Knoll W 1999 Langmuir 15 1337-47
- Hovmöller S, Sjögren A, Wang D N 1988 51 131-61
- Ilk N, Kosma P, Puchberger M, Egelseer E M, Mayer H F, Sleytr U B, Sára M 1999 J Bacteriol at press
- Jaenicke R, Welsch R, Sára M, Sleytr U B 1985 Bio Chem Hoppe-Seyler 366 663-70
- Küpcü S, Sára M, Sleytr U B 1995 Biochim. Biophys. Acta 1235 263-269
- Küpcü S, Sleytr U B, Sára M 1996 J. Immunol. Methods 196 73-84
- Messner P, Pum D, Sleytr U B 1986a J Ultrastruct Mol Struct Res 97 73-88
- Messner P, Pum D, Sára M, Stetter K O, Sleytr U B 1986b J. Bacteriol. 166 1046-1054
- Müller D J, Baumeister W, Engel A 1996 J Bacteriol 178 25-30
- Müller D J, Schoenenberger C A, Schabert F, Engel A 1997 J Struct Biol 119 149-57
- Müller D J, Fotiadis D, Engel A 1998 FEBS Lett 430 105-11
- Neubauer A, Hödl C, Pum D, Sleytr U B 1994 Anal. Lett. 27 849-65
- Neubauer A, Pum D, Sleytr U B, Klimant I, Wolfbeis O S 1996 Biosens. Bioelectron 11 315-23
- Pum D, Sára M, Sleytr U B 1989 J Bacteriol 171 5296-303.
- Pum D, Weinhandl M, Hödl C, Sleytr U B 1993 J. Bacteriol. 175 2762-66
- Pum D, Sleytr U B 1994 Thin Solid Films 244 882-86
- Pum D, Sleytr U B 1995a Supramol. Sci. 2 193-97
- Pum D, Sleytr U B 1995b Colloids Surf. A: Physicochem. Eng. Aspects 102 99-104
- Pum D, Stangl G, Sponer C, Fallmann W, Sleytr U B 1996 Colloids Surf. B: Biointerfaces 8 157-62.
- Pum D, Stangl G, Sponer C, Riedling K, Hudek P, Fallmann W, Sleytr U B 1997 Microelectron. Eng. 35 297-300
- Pum D, Sleytr U B 1999 Trends in Biotechnol 17 8-12
- Ries W, Hotzy C, Schocher I, Sleytr U B 1997 J Bacteriol 179 3892-8
- Sackman E 1996 Science 271 43-8
- Sára M, Sleytr U B 1989 Appl. Microbiol. Biotechnol. 30 184-189
- Sára M, Egelseer E M 1996 Functional aspects of S-layers (Crystalline bacterial cell surface proteins) ed U B Sleytr, P messner, D Pum, M Sára (Austin: R G Landes Comp.) p 103
- Sára M, Küpcü S, Sleytr U B 1996 Biotechnological applications of S-layers (Crystalline bacterial cell surface proteins) ed U B Sleytr, P messner, D Pum, M Sára (Austin: R G Landes Comp.) p 133
- Sára M, Egelseer E M, Dekitsch C., Sleytr U B 1998 J Bacteriol 180 6780-83
- Schuster B, Pum D, Sleytr U B 1998a Biochim Biophys Acta Biomembr 1369 51-60
- Schuster B, Pum D, Braha O Bayley H, Sleytr U B 1998b Biochim Biophys Acta Biomembr 1370 280-8
- Shaw J, Hatzkis M, Babich E, Paraszcak J, Whitman D, Steward K 1989 J. Vac. Sci. Technol. B7 1709
- Shenton W, Pum D, Sleytr U B, Mann S 1997 Nature 389 585-87
- Sleytr U B 1975 Nature 257 400-2
- Sleytr U B, Messner P 1983 Ann Rev Microbiol 37 311-39
- Sleytr U B, Messner P 1989 Electron Microscopy of Subcellular Dynamics ed H Plattner (Boca raton: CRC Press)
- Sleytr U B, Messner P, Pum D, Sára M 1996 Crystalline bacterial cell surface proteins (Austin: R G Landes Comp)
- Sleytr U B, Pum D, Fallmann W, Stnagl G, Löschner H. 1997 Austrian patent 373/97
- Sleytr U B, Messner P, Pum D, Sára M 1999 Angew Chemie Int Ed 38 1034-54
- Sleytr U B, Sára M, Pum D 2000 (Supramolecular Polymerization) ed A Ciferri (New York: Marcel Dekker) at press
- Taga K, Kellner R, Kainz U, Sleytr U B 1994 Anal. Chem. 66 35-9
- TenWolde A 1998 Nanotechnology: Towards a molecular construction kit (The Hague: STT)
- Weiner C, Sára M, Sleytr U B 1994 Biotechnol. Bioeng. 43 321-30
- Wetzer B, Pum D, Sleytr U B 1997 J. Struct. Biol. 119 123-28
- Wetzer B, Pfandler A, Györvary E, Pum D, Lösche M, Sleytr U B 1998 Langmuir 14 6899-6906
- Weygand M, Wetzer B, Pum D, Sleytr U B, Cuvillier N, Kjaer K, Howes P B, Lösche M 1999a Biophys. J. 76 458-68
- Weygand M, Schalke M, Howes P B, Kjaer K, Friedmann J, Wetzer B, Pum D, Sleytr U B, Lösche M 1999b J. Mater. Chem. at press
|