Theoretical analysis of a carbon-carbon dimer placement tool for diamond mechanosynthesis
Zyvex,
Richardson, Texas 75081 USA
This is an abstract
for a presentation given at the
10th
Foresight Conference on Molecular Nanotechnology
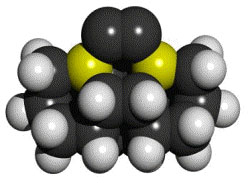 Arranging atoms in most of the ways permitted by physical law is a fundamental objective of nanotechnology. A more modest and specific objective is the ability to synthesize a wide range of stiff hydrocarbons – including molecularly precise diamond structures – using positionally controlled molecular tools. Such positional control might be achieved using an instrument like a Scanning Probe Microscope (SPM) or in the future by a molecular-scale robotic arm. Arranging atoms in most of the ways permitted by physical law is a fundamental objective of nanotechnology. A more modest and specific objective is the ability to synthesize a wide range of stiff hydrocarbons – including molecularly precise diamond structures – using positionally controlled molecular tools. Such positional control might be achieved using an instrument like a Scanning Probe Microscope (SPM) or in the future by a molecular-scale robotic arm.
Several theoretical proposals for molecular tools have already been made [1-4]. In this paper, we propose and analyze new tools for the precise placement of two carbon atoms (a carbon dimer) on a growing molecular structure. Two carbon atoms held together by a triple bond can – for many purposes – be treated as a single unit: a dimer. The function of a dimer placement tool is to position the dimer, then bond the dimer to a precisely chosen location on a growing molecular structure, and finally to withdraw the tool – leaving the dimer behind on the growing structure. To achieve this, the dimer is required to be (1) bonded relatively weakly to the tool and (2) highly strained and thus highly reactive so it will strongly bond to the growing molecular structures to which it is added.
There is a large combinatorial space of possible tools that might satisfy both requirements. For the proposed tools that are modeled computationally here, the requirements are met by bonding the dimer to two supporting atoms selected from the elements silicon, germanium, tin or lead (which form progressively weaker bonds to carbon). The supporting atoms are part of two substituted adamantane frameworks that position and orient them. The two substituted adamantane frameworks are rotated and fused together, creating very high angle strain in the bonds between the two supporting atoms and the dimer.
Density Functional Theory (DFT) analysis suggests that this class of highly strained and reactive tools is stable prior to interacting with any surface, and remains relatively weakly bonded to the dimer while still orienting the dimer appropriately. Each desired tool structure is a minimum on the Potential Energy Surface (PES). Several possible undesired alternative tool structures are transition states or are not even stationary points on the PES.
References
- Charles B. Musgrave, Jason K. Perry, Ralph C. Merkle, William A. Goddard III, "Theoretical studies of a hydrogen abstraction tool for nanotechnology," Nanotechnology 2(1991):187-195; http://www.zyvex.com/nanotech/Habs/Habs.html.
- K. Eric Drexler, Nanosystems: Molecular Machinery, Manufacturing, and Computation, John Wiley & Sons, New York, 1992; http://www.zyvex.com/nanotech/nanosystems.html.
- Ralph C. Merkle, "A proposed 'metabolism' for a hydrocarbon assembler," Nanotechnology 8(1997):149-162; http://www.zyvex.com/nanotech/hydroCarbonMetabolism.html.
- 4. Stephen P. Walch, Ralph C. Merkle, "Theoretical studies of diamond mechanosynthesis reactions," Nanotechnology 9(1998):285-296.
Abstract in Microsoft Word® format 48,966 bytes
*Corresponding Address:
Ralph C. Merkle
1134 Pimento Ave, Sunnyvale, CA 94087 USA
Phone: 408-730-5224 Fax: 408-730-5219
Email: [email protected]
Web: http://www.merkle.com
|