Behaviour of individual microtubules and microtubule bundles in electric fields
W. Vatera, R. Strackea, K.J. Böhma, C. Speicherb, P. Weberb, E. Unger*, a
aInstitute of Molecular
Biotechnology, Beutenbergstrasse 11, D-07745 Jena, Germany
bFraunhofer Institute
for Biomedical Engineering, Ensheimer Straße 48, D-66386 St. Ingbert,
Germany
This is an abstract
for a presentation given at the
Sixth
Foresight Conference on Molecular Nanotechnology.
There will be a link from here to the full article when it is
available on the web.
Introduction
Microtubules (MTs) are cylindrical organelles of eukaryotic cells with diameters of about 25 nm and lengths preferentially in the micrometer range. They consist of 5-nm protofilaments, built up by longitudinally associated ab-tubulin dimers. The strict alternation of a- and b-subunits results in a longitudinal polarity. That means that a protofilament starts with a-tubulin and terminates with b-tubulin. Within the MT, the protofilaments are laterally associated with same polarity. Consequently, at one end of a MT a-tubulin and at the opposite one b-tubulin is found, exclusively. MTs are involved in several cellular mechanisms, as segregation of chromosomes, maintenance of cell shape, and intracellular transport of vesicles. Because of their abundance in neuronal tissues, their constituting proteins (MT proteins = tubulin + MT-associated proteins) can be easily isolated from brain with high yields (Shelanski et al., 1973). Under certain conditions, MT proteins can reconstitute MTs in vitro. Therefore, the reassembly of MT protein can be successfully used as a potent tool in screening for MT-affecting drugs, including cancerostatics (Dustin, 1984). Moreover, the potency of tubulin to self-assemble in vitro into MTs and numerous polymorphic assemblies with protofilament character provides the basis for formation of different biomaterials of nanometer scale (Unger et al., 1990). There are already first papers indicating that MTs could play an important role in nanotechnological applications (Kirsch et al., 1997; Hofinger et al., 1997; Fritzsche et al., 1998). One important step in this regard is to align a large number of MTs, especially with parallel and unipolar structural orientation. Its realization would enable the production of large grids with periodicities in the 25-nm range. Moreover, unipolar arranged MTs fixed on a flat support would be a prerequisite for kinesin- or dynein-driven motility machineries (nanomotors).
A way to align MTs could be the application of electric fields. Because of the intrinsic electric polarity of the tubulin dimer (Tuszynski et al, 1995), MTs have a permanent dipole moment and should be aligned due to an external, static electric field. Previous investigations of Vassilev et al. (Vassilev et al., 1982, 1983, 1985) suggested that it is experimentally possible to align single MTs by application of weak electric fields.
We have inspected the effects of electrical fields both on individual MTs in solution with low MT protein concentrations (< 0.1 mg/ml), using differential interference contrast microscopy, and on MT clusters (bundles) which are formed spontaneously in high concentrated solutions (> 5 mg/ml), applying polarization optical methods.
Material and Methods
Microtubule preparation
MT protein was isolated from fresh porcine brain by two temperature-dependent disassembly/reassembly cycles according to Shelanski et al. (1973), with slight modifications (Vater et al. 1983). A buffer A, consisting of 20 mM PIPES, 80 mM NaCl, 1 mM EGTA, 0.5 mM MgCl2, 1 mM DTT, pH 6.8, was used for all steps. For MT reassembly, buffer A was supplemented with 1 mM GTP. After two cycles the MT protein was again reassembled for 10 min at 37°C followed by 10 min incubation with 20 µM taxol for MT stabilization. These taxol-stabilized MTs were frozen and stored at -80°C until use.
Interference contrast microscopy
For observation of individual MTs, we used a device mounted on a microscopic slide: The upper side of the slide was covered by double-adhesive tape (25 mm thick) from which a stripe 2 mm in width and about 50 mm in length was cut, according to Fig. 1b.

Full size Figure 1
Fig. 1: Chamber for microscopic observation of MTs in electric fields. a - side view, b - top view; 1 - glass slide, 2 - cover slip, 3 - buffer reservoir, 4 - platinum electrode, 5 - observation channel, 6 - hole in the cover slip.
A cover slip 24 mm x 60 mm with two holes of about 1 mm diameter, drilled by a diamond tool, was attached on the double-adhesive tape. In this way, a channel 2 mm wide and 25 mm high results between the supporting glass slide and the cover slip. Two plastic blocks with a central drill hole as buffer reservoirs were attached onto the cover slip at the site of the holes also by double-adhesive tape and sealed by paraffin. Platinum wires in the plastic blocks provide the electric contact from the power supply to the buffer reservoirs (see Fig. 1a).
A MT suspension (< 0.1 mg/ml protein) was filled in the channel using one of the holes in the cover slip. To prevent fluid flow due to unequal levels of the buffer in both reservoirs, the holes in the cover slip were sealed by agarose gel, maintaining the passage of the electric current through the channel. After filling the buffer reservoirs and connecting the power supply the device was ready for use. The voltage could be varied between 0 to 100 V dc, resulting in a maximum field strength of 20 V/cm. For microscopic observation a Zeiss Axiophot microscope in DIC-mode with video contrast equipment was used.
Glass cuvette between crossed polarizers
The experimental set-up is characterized by a 5-mW He-Ne-Laser (632.8 nm), two crossed optical linear polarizers, a 1-cm glass cuvette, two copper plate electrodes tightly attached to the outer sides of the cuvette, a voltage supply (130 - 200 V dc), and a Si-photodiode with amplifier. The cuvette was filled with taxol-stabilized MTs (> 5 mg/ml protein). In this device, the alignment of the birefringent MTs can be detected by the increase of photodiode voltage.
Polarization microscopy in thin electric cells
We have placed a thin chamber within a polarisation microscope. The chamber consists of a microscopic slide on which two isolated parallel copper wires are mounted (diameter about 0.3 mm, distance 2 mm, Voltage: 0.5 - 30 V). 50 µl MT solution were placed onto the slide and closed with a cover slip.
Results and Discussion
Interference contrast microscopy
Without electric field, the suspended MTs showed only a Brownian motion with a random axis orientation. In the electric field, MT movement from the negative pole to the positive pole took place immediately, indicating a negative net charge of the macromolecules. However, under the chosen conditions (maximum field strength of 20 V/cm) the moving MTs could not be parallel arranged. The supposed alignment by electric field-dipole interaction was most likely prevented by statistic thermal motion. At a higher field strength, the MTs were found to tend to be destroyed, apparently due to segregation of ionic components in the buffer. In addition, field-induced MT movement was modified by the electroosmotic effect, i.e., near the glass surface a liquid flow from the positive to the negative pole was observed, affecting MT movement.
Investigations in glass cuvettes between crossed polarizers
Typically, the voltage (alignment) curve (Fig. 2) was characterized by a strong increase during the first 20 - 40 min (maximum 4 V). Thereafter, the voltage decreased again and reached after further 100 - 120 min the value measured at the beginning of the action of the electric field. The effect could be reactivated (after stirring the suspension), but the previous maximum value was not reached again (data not shown). Without electric field, the voltage varied only in a range between 0.12 - 0.14 V. A certain value of applied electric field (> 50 V/cm) was strictly necessary, otherwise orientation effects could not be observed.
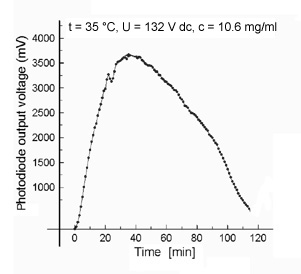
Fig. 2: Typical course of photodiode output in the case of concentrated MT suspensions in the electric field.
The increase of the measured photodiode voltage output apparently reflects a MT alignment in the electric field. However, the slow kinetic of the alignment is surprising. Since highly concentrated MT solutions are not homogeneous, we suggest that MT clusters were already formed prior to field application and that these clusters will be slowly aligned.
We suppose that a (slowly preceding) separation of buffer ions might be responsible for the decrease of the signal. Local changes in the buffer compositions could cause MT disassembly. On the other hand, buffer ion separation should also induce changes in the local electric field (compensation effects). Both assumptions are consistent with the observed reactivation of the alignment after stirring (reassembly and/or realignment).
Polarization microscopy in thin electric cell
MT clustering in highly concentrated solution was verified by polarization microscopy. Already without electric field permanent slow changes of the arrangement of the MT bundles took place. Brownian motion could not be observed (in contrast to the investigations on single MTs), obviously because of the high viscosity in the concentrated MT suspension. Switching on the electric field did not cause an instantaneous effect. However, after about 30 min some MT bundles disappeared under these conditions.
Conclusion
Our results suggest that MTs can be aligned in electric fields. This might contribute to basic solutions of positioning MTs on supports in a parallel and unipolar manner for nanotechnological applications. However, several technical details remain to be solved for improvement the degree of ordering.
References
- Dustin, P. (1984). Microtubules. Springer Verlag, Berlin, Heidelberg, New York, Tokyo
- Fritzsche, W., Böhm, K. J., Unger, E. and Köhler, M. (1998). Making electrical contact to single molecules. Nanotechnology, in press
- Hofinger, J., Kirsch, R., Mertig, M., Pompe, W., Unger, E. and Wahl, R. (1997) Metallische Nanostruktur auf Basis selbstassemblierter, geometrisch hochgeordneter Proteine sowie Verfahren zu deren Herstellung. Wo 9748837, 24.12.97
- Kirsch, R., Mertig, M., Pompe W., Wahl, R., Sadowski, G., Böhm, K.J. and Unger, E. (1997). Three-dimensional metallization of microtubules. Thin Solid Films, 305, 248-253.
- Shelanski, M.L., Gaskin, F. and Cantor, C. R. (1973). Microtubule assembly in the absence of added nucleotides. Proc. Natl. Acad. Sci. USA, 70, 765-768
- Tuszynski, J.A., Hameroff, S.H., Sataric, M.V., Trpisova, B.T. and Nip, M.L.A. (1995). Ferroelectric behaviour in microtubule dipole lattices: implications for information processing, signalling and assembly/disassembly. J. Theor. Biol., 174, 371-380
- Unger, E., Böhm, K.J. and Vater, W. (1990). Structural diversity and dynamics of microtubules and polymorphic tubulin assemblies. Electron Microsc. Rev., 3, 355-395
- Vassilev, P.H., Dronzin, R.T., Vassileva, M.P. and Georgiev, G.A. (1982). Parallel arrays of microtubules formed in electric and magnetic fields. Biosci. Rep., 2, 1025-1029
- Vassilev, P., Dronzin, Valevski, G. and Kanazirska, M. (1983). In vitro polymerization of tubulin modified by application of low-intensity electric and magnetic fields. Stud. biophys., 94, 139-140
- Vassilev, P. and Kanazirska, M. (1985). The role of cytoskeleton in the mechanisms of electric field effects and information transfer in cellular systems. Med Hypotheses, 16, 93-96
- Vater W., Böhm K.J. and Unger E. (1983). Effects of DNA on the taxol-stimulated in vitro assembly of microtubule protein from porcine brain. Stud. biophys., 97, 49-60
*Corresponding Address:
Prof. Dr. Eberhard Unger
IMB Institute of Molecular Biotechnology, Department of Molecular Cytology - Electron Microscopy
Beutenbergstrasse 11, D-07745 Jena, Germany
Tel.: **49 (3641) 65 6160; Fax: **49 (3641) 65 6166
Email: [email protected]; Web:
http://www.imb-jena.de/www_elmi/
|