Towards a Light Adressable Transducer Bacteriorhodopsin-based
1 El.B.A.
Foundation, Via Giotto 2, Genova 16153 Italy
2 Polo Nazionale Bioelettronica - Parco
Scientifico e Tecnologico dell'Elba,
Via Roma 28, 57030 Marciana (LI), Italy
3 Institute of
Biophysics, University of Genova, Via Giotto 2,
Genova 16153 Italy |
This is a draft paper
for a talk at the
Fifth
Foresight Conference on Molecular Nanotechnology.
The final version has been submitted
for publication in the special Conference issue of Nanotechnology.
This page uses the HTML <sup> and <sub>
conventions for superscripts and subscripts. If "103"
looks the same as "103" then your browser does not
support superscripts. If "xi" looks the
same as "xi" then your browser does not support
subscripts. Failure to support superscripts or subscripts can
lead to confusion in the following text, particularly in
interpreting exponents.
Abstract
Highly oriented bacteriorhodopsin films were deposited by
means of a specially designed procedure of electric field
assisted monolayer engineering. The self-assembly of the
monolayer at the air/water interface was controlled by the
increase of the surface pressure. The monolayers were transferred
onto solid substrates by Langmuir-Schaefer technique. Electrical
measurements on the films with a specially designed chamber
confirmed the improved orientation of the films. Possible
application of the films in practical devices such as biosensor
transducer is discussed, keeping in mind features and inherent
limitations when compared with existing silicon transducers.
Introduction
During last years the understanding of structure and function
of several biological systems has grown rapidly. Among them, the
study of Bacteriorhodopsin (BR) protein and the elucidation of
its function as a light driven proton pump represents one of the
most interesting examples [1-3]. BR is a light-transducing
protein in the Purple Membrane (PM) of Halobacterium Halobium.
Its features allow one to identify and design several potential
bioelectronic applications aimed to interface, integrate or
substitute the silicon based microelectronics systems, as well as
to develop molecular devices [4]. BR is a notable exception in
respect with the usual biological molecules, being mechanically
robust, chemically and functionally stable in extreme conditions,
like high temperatures [5-7], which usually represents one of the
key parameters of working conditions. Furthermore, it possesses
remarkable photonic and photovoltaic properties which have been
exploited for molecular device constructions. For these reasons
BR has been adopted as a building block for a number of
experimental prototypes, such as filters, photocells, artificial
photoreceptors, optical memories, image sensors and biosensors
[3, 9-14].
In addition, thin film technologies [15] allow the assembly of
biological materials in a 2D system, usually required for a
device development. Among these technologies, the
Langmuir-Blodgett (LB) one [15-17] seems to be one of the most
promising, due to its ability to form molecular systems having an
high packing degree and a molecular order. Moreover, it has been
possible to assess that such a technique allows the fabrication
of 2D protein closely packed structures, showing an enhancement
of some chemical-physical properties or an induction of new
properties commonly known for proteins in solution or even in
membranes [6, 18-20]. These properties include the long-term
stability to thermal and functional (photochemical) degradation.
Therefore, investigators have shown considerable interest in
the adoption of the Langmuir-Blodgett technique, or its
modifications, to make molecular electronic devices using, in
particular, as an active component, a light-transducing protein
like BR. In fact, the ability of BR to form thin films with
excellent optical properties, and the intrinsic properties
itself, make it an outstanding candidate for use in optically
coupled devices.
BR thin layers have been widely studied [21-25] as they
perform bistability in the optical absorbance and provide
light-induced electron transport of protons through the membrane.
Furthermore, their extremely high thermal and temporal stability
allow to consider them also as sensitive elements for
electrooptical devices [26-25]. However, in order to use BR
properties to provide photovoltage and photocurrent, it is
necessary to orient all the molecules in such a way that all the
proton pathways are oriented in the same direction. LB technique
in its usual version does not allow to realize it. When BR
containing membrane fragments are spread at the air/water
interface, they orient themselves rather randomly in such a way
that proton pathway vector is oriented in opposite directions in
different fragments. Nevertheless, it is known a technique of
electrochemical sedimentation, which allows to deposit highly
oriented BR layers. However, the layers, deposited with this
technique are rather thick and not well controllable in
thickness.
The aim of this work is then to modify the LB technique in
order to obtain highly oriented BR layers and to provide a
suitable solution for the development of a BR-based
nanotransducer.
Materials and Methods
BR used in the work was from Sigma. Films were deposited on
Langmuir trough (MDT, Moscow, Russia). Water used in the work was
purified by Milli-Q system till the resistivity 18.2 M[Omega] cm.
NaCl and KCl were also from Sigma. Millipore filters with the
hole diameter of 0.10 � were used for the film deposition.
Film structure was studied with small-angle X-ray
diffractometer with position sensitive detector (AMUR-K) at the
Elba Foundation laboratory in Genova and on small-angle X-ray
station at the Elettra Synchrotron in Trieste (Italy).
Film formation
Langmuir-Blodgett (LB) technique allows to form a
monolayer at the water surface and to transfer it to the surface
of supports. Formation of the BR monolayer at the air/water
interface, however, is not a trivial task, as it exists in the
form of membrane fragments. These fragments are rather
hydrophilic and can easily penetrate the subphase volume. In
order to decrease the solubility, the subphase usually contains a
concentrated salt solution. It was already shown the efficiency
of the film deposition by this approach [29]. Nevertheless, it
does not allow to orient the membrane fragments. As the
hydrophilic properties of the membrane sides are practically the
same, fragments are randomly oriented in opposite ways at the
air/water interface. Such film cannot thereby be useful to this
work, as the proton pumping in the transferred film will be
compensated. On the other hand the technique of electrochemical
sedimentation is known to form rather thick BR films by orienting
them in the electric field.
Therefore, the following method was suggested and
realized in this work (scheme is shown in the Figure 1). 1.5 M
solution of KCl or NaCl (the effect of preventing BR solubility
of these salts is practically the same) was used as a subphase.
Platinum electrode was placed in the subphase. Flat metal
electrode, with an area of about 70% of the open barrier through
area, was placed at about 1.5 - 2 mm upper the subphase surface.
Negative potential of 50-60 V was applied to this electrode with
respect to the platinum one. BR solution was injected with
syringe into the water subphase in dark conditions. The system
was left in the same conditions for electric field induced
self-assembling of the membrane fragments during 1 hour. After
this, the monolayer was compressed till 25 mN/m surface pressure
and transferred onto the substrate (porous membrane). The
residual salt was washed with water. The water was removed with
the nitrogen jet.
Figure 1. Scheme of the electric field assisted BR monolayer
formation.
Photoresponse measurements
Photoresponse was studied with a home made working chamber.
The filter with deposited BR film was placed between two aqueous
solutions (buffered with the addition of KCl). It was fixed with
a rubber ring to prevent the leakage of the solutions from one
section to the other. Photocurrent was measured between these
sections with 6517 electrometer (Keithley). Illumination was
carried out with a usual tungsten lamp through a fibre optic
lightguide. The chamber was placed into the metal box to be
protected from electrical noise and light.
Light illumination resulted in the sharp increase of the
current through the membrane up to 1000 pA, while without
illumination the noise current was of about 10 pA. It is worth to
mention that over up to twenty experiments carried out over one
year period with the same original BR preparation properly
stored, only about a half of the prepered samples allowed to
register the above mentioned photocurrent. Several experimental
factors appear indeed to negatively influence the resulting
photocurrent, namely age of the BR, film defects due to the
porous nature of the supporting substrate and the experimental
configuration.
Results and Discussion
The dependence of the surface pressure upon the
time with and without applied electric field is shown in Figure
2. It is clearly visible, that electric field improve strongly
the ability of the membrane fragments to form a monolayer at the
water surface.
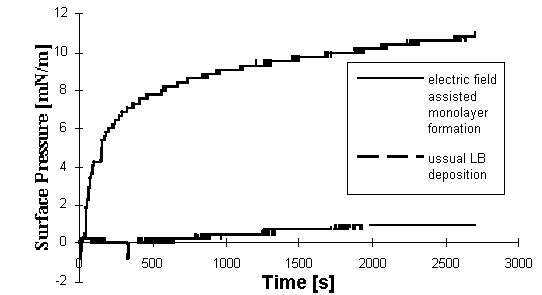
Figure 2. Dependence of the surface
pressure of BR monolayer upon the time in presence and
absence of the electric field.
X-ray measurements of the deposited multilayers
revealed practically the same structure in films prepared with
usual LB technique and electric field assisted monolayer
formation. Indeed, electric field only aligns the fragments at
the air/water interface, providing equal orientation of the
proton pathways. Layered structure in this case remains the same.
X-ray curves (Fig. 3) from both types of samples revealed Bragg
reflection corresponding to the spacing of 46 , what is in a good
correspondence with the membrane thickness.
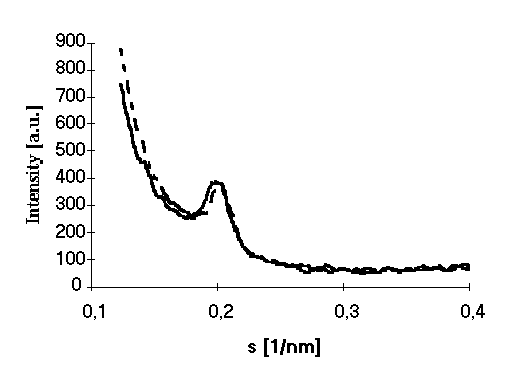
Figure 3. X-ray pattern of films prepared
with (dotted line) and without (solid line) electric field
assisted self assembling.
Furthermore, as previously shown (13, 18, 20,
21), with the LB technique the heat stability of the BR mutilayer
at 25 mN/m surface prssure apperas significantly improved with
respect to both the solution and the self-assembly (Figure 4).
Table 1 Mean value of phototocurent observed in the
system using porous membranes covered with BR film deposited
by ussual LB technique and electric field assisted. A
standard error of about 10 percent is observed over five
independent positive measurements.
| |
light on current [pA] |
light off current [pA] |
| usual LB technique |
15 |
10 |
| electric field assisted monolayer formation |
820 |
10 |
In order to control the degree of BR orientation
photo induced current was measured in the described measuring
chamber. 1 monolayer of BR was deposited onto the porous
membrane. The results are summarized in the Table 1. It is clear,
that the photoresponce in the case of electric field assisted
monolayer formation is much higher with respect to that after a
normal LB deposition (in the last case the signal value is
comparable with the noise, indicating a mutually compensating
orientation of the membrane fragments in the film). The observed
results allow to conclude, that the suggested method of
electrically assisted monolayer formation is suitable for the
formation of BR LB films, where the membrane fragments have
preferential orientation.
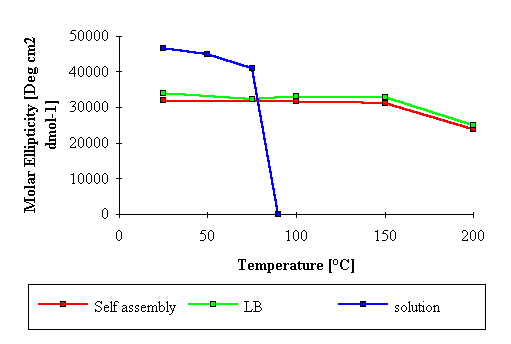
Figure 4 Molar ellipticity versus
temperature for BR multilayers prepered by LB technique
(solid line) and by self-assembly (dotted line). The
corresponding value for BR in solution is also given
(dotted-solid line).
As the electric field assisted monolayer formation at the
air/water interface turned out to provide highly oriented BR
LB film formation, it appears possible to suggest one new
application of BR films as transducer. The principles of the
nanodevice realization are described below.
Device principle
The scheme of the proposed device is presented in
Figure 5. Porous membrane with deposited BR film is separating
two chambers with electrolytes. Light fibre is attached to the
X-Y mover, which allows to illuminate desirable parts of the
membrane. Illumination of the membrane part will result in the
proton pumping through it, carried out by BR. Therefore, a
current between the electrodes will appear. This current must
depend upon several factors, such as light intensity, pH of the
electrolytes and gradient of the pH on the membrane. One of the
possible applications of the suggested device is mapping of 2D pH
distribution in the measuring chamber, which can result from the
working of enzymes, immobilized in this chamber. By scanning the
light over the membrane it will be possible to obtain the current
proportional to the pH gradient in the illuminated point and,
maintaining the pH value fixed in the reference chamber, it will
be possible to calculate absolute pH values in different points
over the whole membrane surface. If different types of enzymes,
producing or consuming protons during their functioning, will be
distributed over the area closed to the membrane, the device will
allow to determine the presence of different substrates in the
measured volume, performing, therefore, a multienzymatic
biosensing.
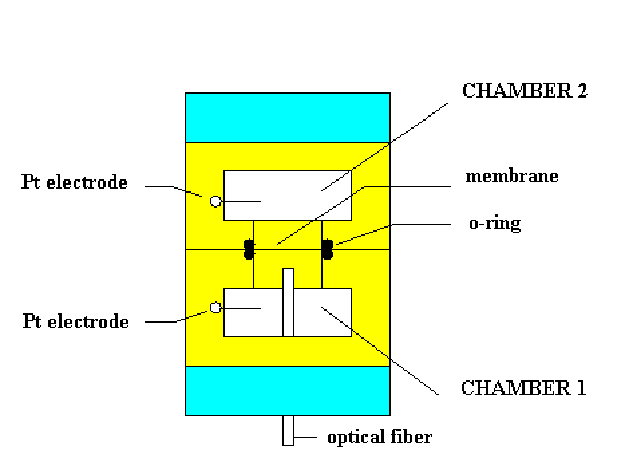
Figure 5. Schematic view of the measuring
chamber used for the experiment with the BR membrane.
Space resolution of the transducer, in principle, is
extremely high. As each BR molecule performs proton pumping,
it will be comparable with the protein size (about 2 nm). In
practice, however, it will be limited by the technical
difficulty in focusing the light beam at such high
resolution, but, in any case, it will be more than in any
existing transducer.
Comparison with the existing devices
Several silicon-based biosensors have been
developed for various applications, such as cell metabolism or
immunoenzymatic activity determination. Due to its performances
and its structural simplicity one of most attractive transducers
based on a silicon heterostructure is the Light Addressable
Potentiometric Sensor (LAPS) [30,31]. It consist essentially of a
silicon substrate coated, on the front side, with an insulating
layer. This layer is in direct contact with the solution to be
analyzed. The sensitive layer of the device consists of Si3N4. A
light source illuminates the rear or the front side of the
transducer, while an electronic circuit (namely a potentiostat)
bias the structure. In its main configuration, LAPS acts as a pH
meter, able to detect a pH variation in a microenvironment. In
appropriate conditions a photocurrent flow through the system and
a pH variation is registered as a phocurrent displacement.
Moreover, it is very attractive the possibility to address
different sensitive zones on the chip, i.e. to perform a pH
mapping [32] of the solution in the chamber, which points out
LAPS as the best candidate for comparison with the suggested
BR-based nanodevice.
For a comparison purpose, the addressability of
LAPS should be considered, since it represents one of the most
attractive features of both systems. LAPS are usually addressed
by means of light coming either directly from a light emitting
diode or through an optical fiber. The light impinging the
silicon substrate causes the generation of hole-elctron pairs,
which diffuse in the silicon bulk. Those pairs which reach the
electric field present under the oxide region are separated, and
the minority carriers give rise to a photocurrent, which is
indeed the measuring signal. Since the carriers diffusion is
three dimensional, multiple light spots can interfere each
other ; in other words the hole-electron pairs generated by
a spot can diffuse into the region downstanding the adjacent
spot, being then separated by the electric field of that region
[31]. This fact, macroscopically, causes interference between the
photocurrents relative to different light excitation sites. The
spatial resolution of LAPS devices depends primarily on the
minority carriers diffusion lenght, defined as :
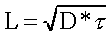
where D is the diffusion coefficient, and [tau]
is the minority carriers lifetime. The lifetime depends strongly
on the silicon substrate [31, 32], and is the major responsible
of the above depicted effect. In practical cases, the distance
between light sources is related to the thickness of the silicon
substrate used. In fact the diffusion in the bulk is isotropic
and we should guarantee that hole electron pairs reach the
space-charge region under the oxide, that is diffuse, more or
less, for a path equal to the silicon thickness.
Therefore the distance between two adjacent light
spots should be larger that two times the silicon thickness
(referring to Figure 6 it must be W > 2 Hsi). One could think
to build LAPS devices based on very thin silicon substrates, but
the counter effect is an increased fragility. In practice, one
can reach a limit spot distance of about 0.2 mm.
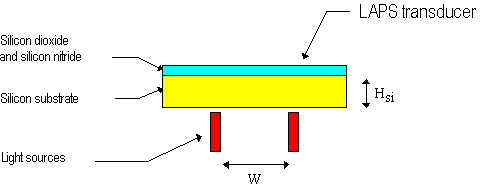
Figure 6. Schematic drawing of a LAPS
devices addressed by multiple light sources.
Even if it is not easy to compare the suggested principle
with already existing devices, some of the characteristics
for both of them are summarized in the Table 2.
Table 2. Typical parameters and features of the
silicon-based LAPS transducer and suggested device.
| |
LAPS |
Suggested device |
| output signal |
current (�A) |
current (nA) |
| illumination light source |
940 nm |
520 nm |
| spatial resolution |
according to the wafer thickness
(0.2 mm in existing devices) |
2 nm in principle but related to
the minimal optical fiber diameter available |
| typical dimensions |
8mm*8mm; 16mm*16mm |
no limits |
| flexibility |
rigid |
very flexible |
| reliability |
months/years |
month/years |
As it is obvious from the table, the suggested device is
comparable and for some aspects even better with respect to LAPS.
These facts allow to conclude, that the device could probably
found applications in the biosensor field as long as the existing
problems could be overcomed. At this stage, however, many
difficulties still remain in the development of this type of
devices with a reproducibility equal to that of silicon-based
ones. In addition, basic problems such as the current detector,
the membrane placement in real cells, the correct addressability
system and even the practical experimental setups for biosensors
should be solved.
Conclusions
Electric field assisted monolayer engineering at the air/water
interface in a Langmuir through as here introduced was proven to
yield reproducable results. Surface pressure measurements
revealed indeed that membrane fragments in such a film are quite
more oriented with respect to usual LB technique deposition. At
the same time significant photoresponse appears present even if
still rather ectic and variabile.
With all due caution the attempt to construct a new usefull
nanodevice based on such elctric-field oriented LB film of
bacteriorhodopsin appears thereby a worthy undertaking, despite
the numerous limitations and problems so far identified.
References
| [1] |
Oesterhelt D., Brauchle C., Hampp N.
(1991), Quaterly Rev. Bioph., 24, 4, 425-478. |
| [2] |
Br[Sinvcircumflex]uchle C., Hampp N.,
Oesterhelt D. (1991) Adv. Mat., 3, 420-428. |
| [3] |
Birge R.R., (1990) Ann. Rev. Phys.
Chem., 41, 683-733. |
| [4] |
Birge R.R., (1992) IEEE Computer, 25,
56-67. |
| [5] |
Hampp N., (1993) Nature, 366, 12. |
| [6] |
Shen Y., Safinya C.R., Liang K.S.,
Ruppert A.F., Rothschild K.J. (1993) Nature, 366, 4850. |
| [7] |
Zeisel D., Hampp N., in Molecular
Manufacturing EL.B.A. Forum Series, Vol. 2 Claudio
Nicolini Ed., 175-188 (1996). |
| [8] |
Oesterhelt D., Br[Sinvcircumflex]uchle
C., Hampp N., (1991) Quaterly Rev. Bioph. 24, 4, 425-478. |
| [9] |
Fukuzawa K., Yanagisawa L., Kuwano H.
(1996) Sensors and Actuators B, 30, 121-126. |
| [10] |
Fukuzawa K. (1994) Appl. Optics, 33
7489-7495. |
| [11] |
Miyasaka T., Koyama K., Itoh I. (1992)
Science, 255, 342. |
| [12] |
Storrs M., Merhl D.J., Walkup J.F.
(1996) Applied Optics, 35, 4632-4636. |
| [13] |
Maccioni E., Radicchi G., Erokhin V.,
Paddeu S., Facci P., Nicolini, C. (1996) Thin Solid Film,
284-285, 898-900. |
| [14] |
Chen Z., Birge R.R. (1993) Trends in
Biotechnology, 11, 292-300. |
| [15] |
Ulman A., An introduction to ultrathin
organic films: from Langmuir-Blodgett to self assembly,
Academic Press: Boston, (1991). |
| [16] |
Roberts G., Langmuir-Blodgett Films,
Plenum Press, New York, (1990). |
| [17] |
Zasadzinski J.A., Viswanathan R., Madsen
L., Garnaes J., Schwartz D.K. (1994) Science, 263,
1726-1733. |
| [18] |
Nicolini, C., Erokhin V, Antolini F.,
Catasti P., Facci P. (1993) Biochimica et Biophysica
Acta, 1158, 273-278. |
| [19] |
Pepe M., Nicolini, C. (1996) J.
Photochem. Photobiol. B., 33, 191-200. |
| [20] |
Maxia L., Radicchi G., Pepe I. |
|