Langmuir-Schaefer Films of
Poly(o-anisidine) Conducting Polymer
for Sensors and Displays
by
Sergio
Paddeu+, Manoj
Kumar Ram†,
Sandro Carrara+, and
Claudio
Nicolini+,†, �
Corresponding Address:
Claudio Nicolini, Fondazione EL.B.A.,
Via Medaglie d'Oro 305 - 00136 Rome, Italy
ph: +39 6 35410728, fax: +39 6 35451637,
email: [email protected]
This is a draft paper
for a talk at the
Fifth
Foresight Conference on Molecular Nanotechnology.
The final version has been submitted
for publication in the special Conference issue of Nanotechnology.
This page uses the HTML <sup> and
<sub> conventions for superscripts and subscripts. If
"103" looks the same as "103"
then your browser does not support superscripts. If "xi"
looks the same as "xi" then your browser does not
support subscripts. Failure to support superscripts or
subscripts can lead to confusion in the following text,
particularly in interpreting exponents.
Abstract
Ultrathin films of polyanilines play an important roles for
the application of this class of polymer in nanotechnology. The
nature of the surface properties is critical for the function of
electronic products which may be determined by the layers of
nanometers thickness of polyanilines. In this regards, the
Langmuir- Schaefer (LS) films of poly(o-anisidine) (POAS)
substituted polyaniline have been fabricated at the molecular
level. The LS films of POAS conducting polymer were investigated
by using Brewster microscopy, ellipsometric and electrochemical
techniques, respectively. The obtained results revealed a regular
deposition during at least 40 monolayers of POAS LS films. The
development of the surface irregularities beyond 40 monolayers of
LS films resulted into an electrochemical kinetics similar to the
electrodeposited films. The electrochemical kinetics in fewer
number of monolayers was shown to be the first electron transfer
process. The nature of anions caused significant changes in the
redox properties of POAS LS films. The electrochromic switching
response time and diffusion coefficient of such LS films were
also estimated by electrochemical investigations. Further, POAS
LS films were used for the detection of HCl in water by
conductrimmetric measurement, which revelaed a detection limit of
0.1 ppm of HCl.
1. Introduction
Polyaniline class of conducting polymer has been widely
investigated aiming towards potential applications for
rechargeable batteries, protection coating, electrochromic
displays, conducting composite material, gas sensor, corrosion
protection, electronic, biosensors and optical devices (1-6). In
fact polyaniline often has been categorized as an intractable
polymer due to the lower processibility (7). In this regard,
efforts have been made in increasing the processibility by using
the subtitutents groups in the monomer or the polymeric backbone
(8-10). Substituted polyanilines such as poly(ortho-toluidine),
poly(methoxy aniline) (POAS), poly(ethoxy aniline) (PEOA) and
poly(methyl aniline) etc. have been synthesized keeping in view
the unusual electroactive properties and improved processibility
(11-12). The substituted polyaniline increases the possibilities
of adopting various techniques in the fabrication of conducting
polymer from thick to ultra thin films, which are appropriate for
several technologies as well as electronic and optical devices
(13-14). In the recent past such ultra thin films of substituted
polyanilines were fabricated by Langmuir-Blodgett (LB) technique
aiming towards its potential application in nanotechnology (15).
In fact, the recent past had also shown the deposition of
quasi -ordered and ordered Langmuir- Blodgett films of
polyaniline, where emeraldine base dissolved in N-methyl
2-pyrrolidinone (NMP) /chloroform (CHCl3) could be used to cast
LB films using an aqueous subphase containing neutral or acidic
water (16-17). The deposited LB films of polyaniline used to give
rise the irregular surface after certain number of deposited
films. The ultra thin films of POAS and PEOA have been recently
fabricated by Langmuir-Blodgett technique (15, 17). POAS LB films
can be considered to be an important class due to their optical
and electrical properties (16). The conductivity and optical
properties of POAS depends upon the oxidation state of the main
chain as well as the degree of protonation of nitrogen atoms in
the polymer backbone. The doping brings the different structure
from emeraldine base to emeraldine salt (16). So, it is an
important factor in knowing about the organization of the polymer
molecules at the air/water interface as well as the physical
properties of the deposited films.
Beginning with this consideration the aim of this paper was to
discuss the various parameters ruling the quality of Langmuir
film as well as optical, electrochemical and structural
properties of POAS LB films. The POAS LB films were studied at
the air/water interface by Brewster angle microscopy (BAM).
Additionally, the deposited films were investigated using cyclic
voltammetry in order to assess the effort of redox states for the
surface morphology in going from thin to thick films. The
electrochromic switching response time at different protonic acid
media was studied on such POAS LS films. The possibility of using
such POAS LS films for the HCl sensor were also performed, which
showed a detection limit of the 0.1 ppm.
2. Experimental details
2.1 Fabrication of POAS LS films
The poly(ortho-anisidine) (POAS) was oxidatively polymerized
using ammonium peroxy-disulphate as reported previously (16, 18).
The emeraldine base (EB) powder was used for the fabrication of
the Langmuir-Schaefer (LS) films. A solution was prepared by
dissolving 1mg of POAS (EB) conducting polymer in 5 ml of CHCl3.
The resulting solution was filtered through the resistant filter
(0.5 m). LB trough [MDT corp.] of size 240 mm x 100 mm and volume
300 ml was used for the fabrication of LS films with a
compression speed maintained at 0.2 mm/sec. The Langmuir trough
was filled with an aqueous subphase containing pH 1 using HCl
acid. 70 l of the solution was spread on a acidic subphase.
Different numbers of monolayers were transferred on to glass
indium-tin-oxide (ITO) plates and [111] silicon substrates by
Langmuir-Schaefer (LS) method at a pressure of 25 mN/m with a
feed back of 5mN/m.
2.2 Ellipsometric measurements
Ellipsometric measurements were performed using a PCSA null
ellipsometer LEPh-2 (Special Design and Production Bureau for
Scientific Devices of the Siberian Branch of the Russian Academy
of Sciences, Novosibirsk) using He-Ne laser (wavelength 632.8
nm). The accuracy of the device was 0.02� with respect to and .
The POAS LS films deposited on [111] silicon substrates
attributed anisotropy, we kept an ideal condition of ellipsoid
position at 70� by properly optimizing our system before the
measurement of the LS films. Various monolayers (from 1 to 60) of
POAS LS films were recorded in the similar condition. The
thickness of the LS films were calculated by using the two layer
model (19).
2.3 Optical measurements
The UV-visible spectra of POAS LS films deposited on quartz
substrates was recorded by using the UV-visible spectrophotometer
(Jasco model 7800).
2.4 Electrochemical measurements
The electrochemical measurements were made by
Potentiostat/Galvanostat (EG & G PARC model 163 with a
software M270). A standard three electrodes configuration were
used, where LS films on glass ITO plate worked as a working
electrode, platinum as a counter and Ag/AgCl as a reference
electrode. The cyclic voltammogram was measured at different scan
rate of POAS LS films of POAS at different concentrations of
protonic acid (HCl).
2.5 Brewster Angle Microscopy
The formation of Langmuir films of POAS, as well as their
deposition onto a glass plates, was analyzed by Brewster Angle
Microscopy (BAM), in particular with a BAM2 microscope (Nanofilm
Technologie Gmbh, G�ttigen, Germany) coupled with a NIMA LB
trough (NIMA, England, type 601; surface pressure sensor type
PS3). BAM images of POAS Langmuir film were acquired during its
formation and after its deposition on quartz plates. The analysis
allowed one to study the morphological features of such films as
well as to reveal the 2D-3D transformations which was taken place
at different surface deposition pressures (20).
3 Results and discussion
3.1 Pressure -area isotherms for POAS Langmuir films
The stability of Langmuir monolayer is somewhat associated to
a high collapse pressure, a steep increase in the surface
pressure curve in the condensed phase and a small hysteresis in
the compression-expansion cycle. The choice of the appropriate
subphase has been proved extremely important for the deposition
of LB film from polyanilines films (16). Fig.1
(curve 1) shows the pressure -area isotherm for the POAS Langmuir
film at pH 6.4. It does not show a steep increase of pressure in
the condensed phase and the yielding (breaking) point for the
pressure has been obtained at a pressure of 42 mN/m. It
attributes that higher pH does not produce good Langmuir
monolayers. So a careful investigation was performed using
different pH of the sub-phases. When the pH is maintained either
5 (curve 2) or 4 (curve 3), no steep increase in the
pressure-area isotherm behavior is obtained. But, when pH of the
medium is lowered (curve 4 to 6) the molecules suffered and are
doped where Langmuir monolayer is more crystalline in nature. The
Langmuir monolayer at lower pH (1 to 3) gives rise to higher
collapse pressure ( 50 mN/m) than higher pH (4 to 6.4). The
importance of doping has been confirmed for the stable
monolayers, which is probably associated with the ordering that
is introduced in the polymer. It can be speculated that the HCl
doping organizes the polymer chain of POAS Langmuir film. The
solid phase transition has started occurring at the molecular
area of 6 �2 between 1 to 3 pH. So an attempt was
made for the estimation of molecular area of POAS at air - water
interface by extrapolating the pressure -area isotherm curve at
different pH of the subphase. It shows that when the pH is
maintained 6.4, the molecular area has been estimated to be 55 �2.
For the calculation of molecular area at condensed phase, one
repeating unit of POAS has been taken into account (16). When the
POAS monolayer is formed at lower pH, the polymer chain orients
and doped with protonic acid. So the area obtained at 1 pH has
been found to be about 21 �2 which should be the
expected area of the aniline monomer. The cross sectional area of
the aniline repeat unit at air - water interface has already
estimated to be 20 �2. So, 25 mN/m pressure has been
carefully selected to deposit the LB films at a subphase pH 1.
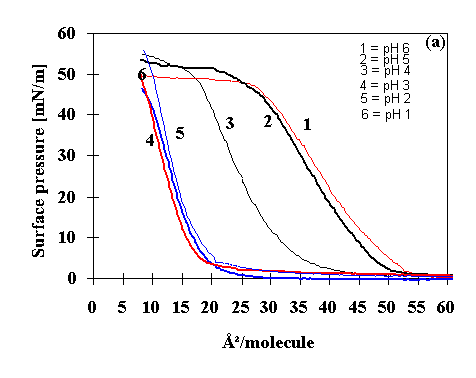
Fig.1 Variation of the pressure vs area
isotherm at different pH using HCl acid
3.2 Films morphology by Brewster Angle Microscopy
The formation of Langmuir films of POAS, as well as their
deposition onto a quartz plates, was analyzed by Brewster Angle
Microscopy (BAM), in particular with a BAM2 microscope (Nanofilm
Technologie Gmbh, G�ttigen, Germany) coupled with a NIMA LB
trough (NIMA, England, type 601; surface pressure sensor type
PS3). The working principle of BAM is based on Fresnel's
principle for polarized light from an interface and it is
described in literature (21). BAM images of POAS Langmuir film
were acquired during its formation and after its deposition. The
analysis allowed one to study the morphological features of such
films as well as to reveal the 2D-3D transformations which was
taken place at different surface deposition pressures. The POAS
Langmuir film formation was analyzed by means of BAM for both ES
forms, which were deposited at pH 1. 2D and 3D transformations
were imaged and correlated registered with the simultaneously p/A isotherm. It was assessed that the
dopant arranged the POAS molecules at the air/water interface in
a different way, as underlined by the decrease of area per
repeating unit (r.u.). This fact underlined that the inclusion of
dopant ions in the molecule, from EB to ES form of POAS polymer,
was able to cause structural and conformational changes due to a
different charge distribution in the backbone. Moreover, the BAM
analysis allowed one to reveal the presence of breaks,
aggregations and collapses. These features represent a crucial
point to be considered during the design of devices based on
highly ordered layered structures with low concentration of
defects. A note fact is that part of such defects are present in
the deposited films which is also already seen at the air/water
interface as shown in Figure 2. Breaks
revealed at the interface (Figure 2 image A) were observed in a
layered structure of POAS (Figure 2 - image C). Furthermore, the
images B and D of figure 2 underlined that the 3D transformations
which was taken place reaching the 2D limiting molecular density,
namely collapses (Figure image B), were a irreversible
phenomenon, as confirmed by the presence of such 3D structures on
deposited POAS films. Breaks (image A) and collapses (image C)
formed at the air/water were revealed also on deposited films of
POAS (images B and D) thus suggesting that such defects were
already present at the air/water interface. A layered structure
of POAS Langmuir layer is displayed in image B: the different
gray levels represent the overlapping of monolayers.
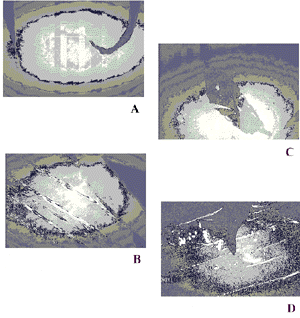
[Full size Figure 2: 58K, 645 x 683 pixels]
Fig.2 Brewster angle microscopy: Image A
(monolayer at air/water interface at 25 mN/m), Image B (monolayer
at air/water interface at 45 mN/m), Image C (monolayer at quartz
plate deposited at 25 mN/m) and Image D (monolayer deposited on
quartz plate at 45 mN/m).
3.3 Ellipsometric studies
The thickness of the deposited POAS LB films at a pressure of
25 mN/m was estimated by ellipsometric measurements. Fig.3 shows the thickness (estimated from
ellipsometric measurements) vs the number of monolayers of POAS
LS films. It reveals a linear fitting measured till 36 monolayers
of the POAS LS films. The thickness obtained for each monolayer
was estimated to be 24 2 �, which resembled with the thickness
measured by x-ray diffraction (16, 19). It attributes that the
each deposited monolayer of POAS molecules are parallel to the
surface (substrate) while measured till 36 monolayers. Whereas,
it attributed a decrease in the thickness of one monolayer layer
calculated from the LS films containing 40 monolayers. Such
decrease in the thickness of each monolayer (after 40 monolayers)
may perhaps be linked to the less transfer of the Langmuir
monolayer molecules or some molecules of POAS are blown in
process of drying under the nitrogen flux. We see the deviation
in linearity for the films deposited after 36-40 monolayers in
Fig.3. So, an attempt was taken to present the two different
slopes for the films deposited till 50 monolayers of POAS LS
films.
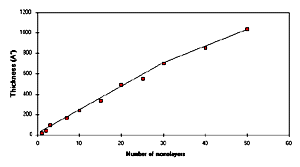
[Full size Figure 3: 5K, 638 x 355 pixels]
Fig.3 Variation of thickness vs number of
monolayers of POAS LS films measured by ellipsometric
measurements.
3.4 UV-visible absorption
Fig.4 (inset) shows the UV- visible
spectra of 20 monolayers of POAS LS films on quartz substrate. It
reveals to the two sharp absorption bands at 348 nm and 750 nm of
the films made by using 1 pH aqueous subphase. The observed peak
at 348 nm attributes to a -* transition centered on the benzoid
ring (inter band transition) and the band seen at 750 nm is due
to the HCl incorporation, when emeraldine base form of POAS
monolayer is formed at pH 1 containing acidic (HCl) subphase
(22). In our earlier work, it was shown that 1 M HCl doping
caused the appearance of 840 nm and 450 nm bands besides the
shift of the bands from 348 nm to 352 nm. The uniformity in
deposition of POAS LS films has also been characterized using
UV-visible absorption spectra. The magnitude of UV-visible
absorption band at 348 nm for number of monolayers is shown in Fig.4. It shows for the increment in absorption
magnitude from one to sixty monolayers of POAS LS films.
Interestingly a linear increase of the UV-visible absorption
magnitude till 37 monolayers can be observed. The deviation in
the linearity in the UV-visible absorption magnitude can be
observed nearly at around 40 monolayers of LS films. So, attempts
were made in showing the two different slope of the number
exceeding than 37 monolayers, which resembles to the
ellipsometric measurements.
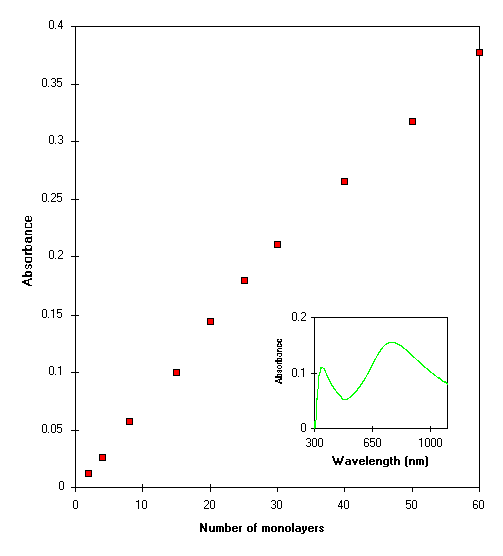
Fig.4 Variation of UV visible absorbance at
348 nm vs number of monolayers. Inset show the UV -visible
spectra for the 20 monolayers POAS LS films made at pH 1.
The little change that occurred in drawing the number of
layers in ellipsometric and UV-visible absorption measurements
may be arising due to the fact that the films were deposited on
the two different substrates (silicon and quartz crystal). The
nature of substrates strongly influences the deposition
parameters of Langmuir monolayers. The above techniques indicate
for the beginning of irregular deposition beyond 40 monolayers.
3.5 Electrochemical studies on POAS LS films
The electrochemical kinetics of various deposited monolayers
of POAS LS films have been studied by performing cyclic
voltammetry in a electrochemical cell consisting of three
electrodes in 1 M HCl medium at a scan rate of 50 mV/sec. Inset
in Fig.5 shows the electrochemical response
of one monolayer of POAS LS film. The first oxidation converts
the film from neutral to polarons states and second oxidation
leads to states of bipolarons. Since the second potential
involves the more electrons than first, we therefore, cannot see
the same peak potential height in one monolayer of POAS LS film.
The CVs of 1 monolayer to 60 monolayers of POAS LS films are
shown in Fig.5. The shape of the cyclic
voltammogram for each number of layers shows the surface confined
species as expected. The cyclic voltammetry peaks are associated
to the oxidation and reduction processes of POAS LS films. The
peak current scale increases linearly as a function of monolayers
as shown in Fig.5. It shows the little change in the value of
peaks potential in going from monolayer to multilayers
deposition. In fact, this shift in the peak potential can be
related to the increase in the more polarons/bipolarons states
for the number of monolayers of POAS LS films. The oxidation and
reduction peaks potential of 10 monolayers in Fig.5 (curve 3)
have the reduction peaks at around 724, 436, 186 and -4 mV,
whereas the oxidation peaks can be seen at 724, 496 and 282 mV.
It can be attributed that there is a little shift in the
oxidation potential at 720 mV, whereas there is gradual increase
in the reduction potential at 720 mV for the number of
monolayers. The electrochemical response of POAS was practically
same as a function of number of monolayers, besides the oxidation
couple at around 724 mV is affected by changing the number of
monolayers (23). In addition the lower value of electrochemical
reduction potential (at 720 mV) of POAS LS films can also be
noticed in close comparison to the polyaniline films (at 0.8V).
The change in reduction potential value is linked to the higher
electronic density states due to the substitution in the aromatic
ring, which facilitates the protonation and the oxidation of the
amine group. Curve 8 in Fig.5 shows the electrochemical response
of 60 monolayers. It shows the quenching of the peak at 720-740
mV as well as the broadening and decrease in the intensity of the
peak potential at round -20 to 0 mV, which may perhaps be linked
to the irregular deposition and the increase in film thickness.
The widening of the peak potential at 720 mV were also observed
while going from 44 to 60 monolayers. It could also be related to
the inhomogeneity incorporated inside the film in the process of
deposition process beyond 40 monolayers. However, it is worth
drawing attention to the fact that the electroactivity of the
polymer depends on the film thickness. The similar results were
observed for the electrochemical grown polyaniline films having
different thicknesses. The electroactvity is higher in the thin
LS films and decreases in rising the thickness. The widening in
the peak potential value from 44 to 60 monolayers can be seen for
the POAS LS films. The resulted cyclic voltammetric for the lower
number of LS films are linked to more number of oxidation and
reduction peak potentials and faster electrochemical response,
whereas CV of the films containing higher number of monolayers
behave little differently in the electrochemical process. The
film formed by using the pulse potential (potentiodynamic) shows
a similar behavior to the LS films of sixty monolayers. The redox
behavior of POAS deposited electrochemically was well established
by Mello et al (23), including such aspect as the dependence of
pH of the solution and relation to the method of preparation
(23). Though, it may seem analogous that electrochemically
prepared films thicker than multilayer LS films, are also regular
(23). The key factor that determines CV response is surface
regularity. The slowing down of electron transfer process was
also seen by evaluating the half peak potential vs the number of
layers, which shows the irregular deposition beyond 40 monolayers
as observed by UV-absorption and ellipsometric techniques,
respectively
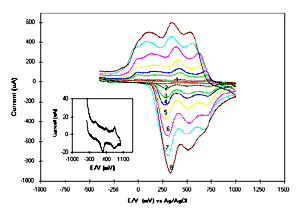
[Full size Figure 5: 11K, 623 x 444 pixels]
Fig.5 Cyclic voltammogram (CV) of POAS LS
films as a function of monolayers viz.: 1 = monolayer, 2 = 5
layers, 3 = 10 layers, 4 = 15 layers, 5 = 25 layers, 6 = 34
layers, 7 = 44 monolayers, 8 = 60 layers; Inset shows the CV of
one monolayer of POAS LS film.
In order to verify, the oxidation and reduction processes of
POAS conducting polymer films and diffusion coefficients, the CVs
of 40 monolayers of POAS LS films were recorded at different
scanned rate. The observed CV response in Fig.6
underlying to the notion that the redox kinetics is probably
controlled by ohmic effect. The gradual change in the peak
potential have been observed, quite similar to the
electrodeposited films. The value of diffusion coefficient (Do)
in different media for POAS LS films was determined using the
Randles -Sevics equation (24, 25). The diffusion coefficient (Do)
has been calculated 1.2 x 10-10 cm2s-1.
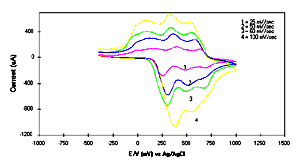
[Full size Figure 6: 7K, 638 x 355 pixels]
Fig.6 CVs of 20 monolayers LS films at
different scan rate as shown in Figure.
The other interesting aspect such as electrochromic effects of
the films were also observed. The electrochromism was noted from
yellow to green and later to violet as the potential was swept
from -0.2 to 1.0 V for such POAS LS films. So the oxidation and
reduction processes in POAS LS films were studied in strong (HCl)
to weak acid (acetic acid) as shown in Figure 7.
These changes are associated to the variations in the electronic
resonant structure of the polymer backbone caused by oxidation
and protonation forms of POAS conducting polymer. The overall
process involves the loss of two electrons and deprotonation for
POAS conducting polymer The oxidation and reduction process and
the lower redox switching of the POAS conducting polymer may be
dependent on redox ionic conductivity of the polymer matrix. It
has been shown that HCl ions show the faster electrochromic
effect (switching half time Hf1/2= 250 ms)
than studied in H2SO4 (Hf1/2=
300 ms) and CH3COOH (Hf1/2 = 1
sec). The oxidation and reduction process in CH3COOH
medium is slower than studied either in H2SO4
or HCl media (26). The weak acid takes time in diffuses to the
POAS films.
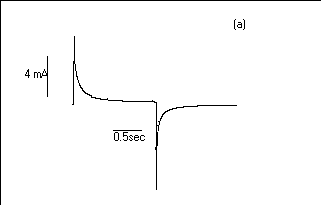
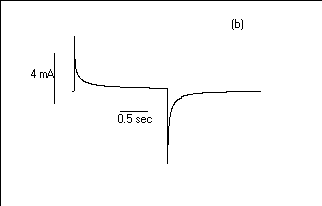
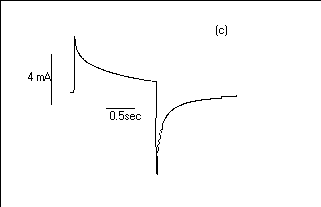
Fig.7 Oxidation and reduction current
response for 30 layers the POAS LS films Viz.:(a) HCl, (b) H2SO4
and (c) CH3COOH media.
3.6 HCl sensing
The HCl sensing in water was performed by depositing 40
monolayers of POAS LB films on the interdigited electrode and the
films was undoped in aqueous ammonia for the five minutes. The
films was washed in water and dried by blowing nitrogen gas. Such
POAS LS films was subsequently dipped at a concentration of HCl
solution for five minutes and current -voltage measurement was
performed. Later, the films was dipped in the higher
concentration for the same time period, dried and I-V
characteristics was measured. This procedure was repeated for
repeated for the higher concentration. The magnitude of the
current measured at 0.5 V was given in Figure 8.
Significant changes in the properties of POAS are observed as the
amount of HCl ions in the solution increases. There is a
continuos increase in the current vs HCl concentration. The
presence of small amount of HCl ions diffuses in to the films and
which can be sensed.
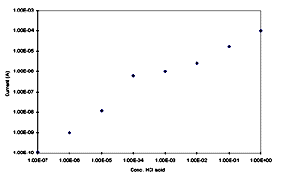
[Full size Figure 8: 5K, 626 x 369 pixels]
Fig.8 Response curve as current vs HCl
concentration.
4. Conclusions
In summary, we have indicated the uniformity of POAS
Langmuir-Schaefer films are restricted to certain number of
monolayers. The thickness of one monolayer estimated from 1 to 40
monolayers is to be 24 , whereas it shows a decrease in thickness
of monolayer measured from 40 to 60 monolayers. It can be noted
that the non-linearity in the ellipsometric or UV-absorption
measurements put forward for the real deposition process.
However, irregularities begin to form after 40 monolayers and the
orderliness in the film decreases, leading to the electrochemical
kinetics of the electrochemical deposited films. The
electrochromism has been studied in the POAS LB films, which
shows a good colour contrast in 40 layers of the films. The HCl
sensor shows the detection limit of 0.1 ppm.
Acknowledgments
We are thankful to Drs. Victor Erokhin, M. Adami, M. Sartore
and M. Salerno for their interesting discussions during the
preparation of the manuscript. Thanks are due to Mr. F. Nozza and
Miss Rando for their help in carrying out the experiments.
Financial supports from EL. B.A. Foundation and the University of
Genoa are gratefully acknowledged.
References
(1) E.M. Genies, P. Hany and C. Santier, 1989 J. Appl.
Electrochem., 18, 751.
(2) M. Pasqualli, G. Pistoia and R. Rosati, 1993 Synth.
Met., 58 1-5.
(3) M. K. Ram, E. Maccioni and C. Nicolini, 1997 Thin Solid
Films, 303 27-33.
84) H. Bleier, J. Finter, B. Hilti, W. Hofherr, C.W. Mayer, E.
Minder, H. Hediger and J.P. Anssermet, 1993 Synth. Metals,
55-57, 3605 - 3610.
(5) J. Pellerino, R. Radebaugh and B.R. Mattes, 1996 Macromolecules,
29 4985.
(6) E. W. Paul, A.J. Ricco and M.S. Wrighton, 1985 J. Phys.
Chem., 89 1441, Y. Echigo, K. Asami, H. Takahashi, K.
Inouue, T. Kabata, O. Kimura and T. Ohsawa, 1993 Synth. Metals,
55-57 3611-3616.
(7) A.J. Epstein and A.G. MacDiarmid, 1991 Chem. Macromol
Sym., 51 217.
(8) E.M. Scherr, A.G. MacDiarmid, S.K. Manohar, J.G. Masters,
Y. Sun, S. Tang, M.A. Druy, P.J. Glatwski, V.B. Cajipe, J.E.
Fischer, K.R. Cromack, M.E. Jozefowicz, J.M. Ginder, R.P. MacCall
and A.J. Epstein, 1989 Synth. Met., 25 649.
(9) J.J. Langer, 1990 Synth. Met., 35 301.
(10) Z.H. Wang, A. Ray, A.G. MacDiarmid and A.J. Epstein, 1991
Phys Rev. B, 43 4373.
(11) M.E. Jozefowicz, A.J. Eipstein, J.P. Pouget, J.G.
Masters, A. Ray, and A.G. MacDiarmid, 1991 Macromolecules, 24
5863
(12) J.P. Pouget, S.L. Zhao, Z. H. Wang, Z. Oblakowski, A.J.
Epstein, S.K. Manohar, J.M. Wiesinger, A.G. MacDiarmid and C.H.
Hsu, 1993 Synth. Metals, 55 -57 341-346.
(13) M. K. Ram, S. Carrara, S. Paddeu, and C. Nicolini, 1997 Thin
Solid Films, 302, 89-97, M.K. Ram, S. Paddeu, S.
Carrara, E. Maccioni and C. Nicolini, 1997 Langmuir, .13,
(10), 2760-2765.
(14) S.V. Mello, L.H.C. Mattoso, R.M. Faria and O.N. Oliveira
Jr., 1995 Synth. Metals, 71 2039-2040.
(15) J.F. Penneau, M. Lapkowski and E.M. Genies, 1989 New
J. Chem., 13 449.
(16) M. K. Ram, N.S. Sundaresan and B.D. Malhotra, 1993 J.
Phys. Chem., 97 11580, M.K. Ram, R. Gowri and B.D.
Malhotra, 1997 J. Appl. Poly. Sci., 63 141-145,
N.E. Agbor, M.C. Petty, A.P. Monkman and H. Harris, 1993 Synth.
Metals, 55-57 3789-3794...
(17) J. H. Cheung, E. Punkka, M. Rikukawa, M.B. Rosner, A.J.
Rorappa, M.F. Rubner, 1992 Thin Solid Films, 210-211
246, A. Dhanabalan, R.B. Dabke, N. Prasant Kumar, S.S. Talwar, S.
Major, R. Lal and A.Q. Contractor, 1997, Langmuir, 13
(16) 4395-4400.
(18) Riul A. Jr., L.H.C. Mattoso, S.V. Mello, G.D. Telles and
O.N. Olieveira, 1995 Synth. Metals, 71 2060
(19) S. Paddeu, M.K. Ram and C. Nicolini, 1997 J.
Phys. Chem.B, 101 4759-4766.
(20) D. Honig, D. Mobius, 1992 Chem. Phys. Lett., 195
50-52
(21) F.A. Jenkins, H.E. White, Fundamental of optics, 4th
ed., MacGraw Hill Book Co., New York, 1987, chapter 24.
(22) J.C. Lacroix, P. Garcia, J.P. Audiere, R. Clement and O.
Kahn, 1991 Synth. Met., 44 117.
(23) S.V. Mello, L.H.C. Mattoso, J.R. Santos Jr., D.
Goncalves, R.M. Faria, O.N. Oliveira, 1995 Electrochimica Acta,
40 12.
(24) M. Verghese, M.K. Ram, H. Vardhan, B.D. Malhotra, S.M.
Ashraf, 1997 Polymer, 38 1625
(25) A.J. Bard, L.R. Faulkner, Electrochemical, Method,
Fundamental and Application, John Wiley, New York, 1980
pp:142.
(26) W. E. Rudzinski, L.Lazano, M. Walker, 1990 ibid. 137
3132.; J.P. Pouget, M.E. Josefowicz, A.J. Epstein, X. Tang, A.G.
MacDiarmid, 1991 Macromolecules, 24 779.
|